Vaccinex's Pepinemab Shows Promise in SIGNAL-AD Trial for Alzheimer's
Vaccinex, Inc., a biotech company in the clinical-stage focusing on Alzheimer’s disease treatment using anti-Semaphorin 4D (SEMA4D) antibody, has disclosed promising outcomes from its early SIGNAL-AD clinical trial evaluating the pepinemab antibody for Alzheimer’s disease. Eric Siemers, MD, Principal Investigator of the SIGNAL-AD trial, shared the primary results during the Alzheimer’s Association International Conference held in Philadelphia.
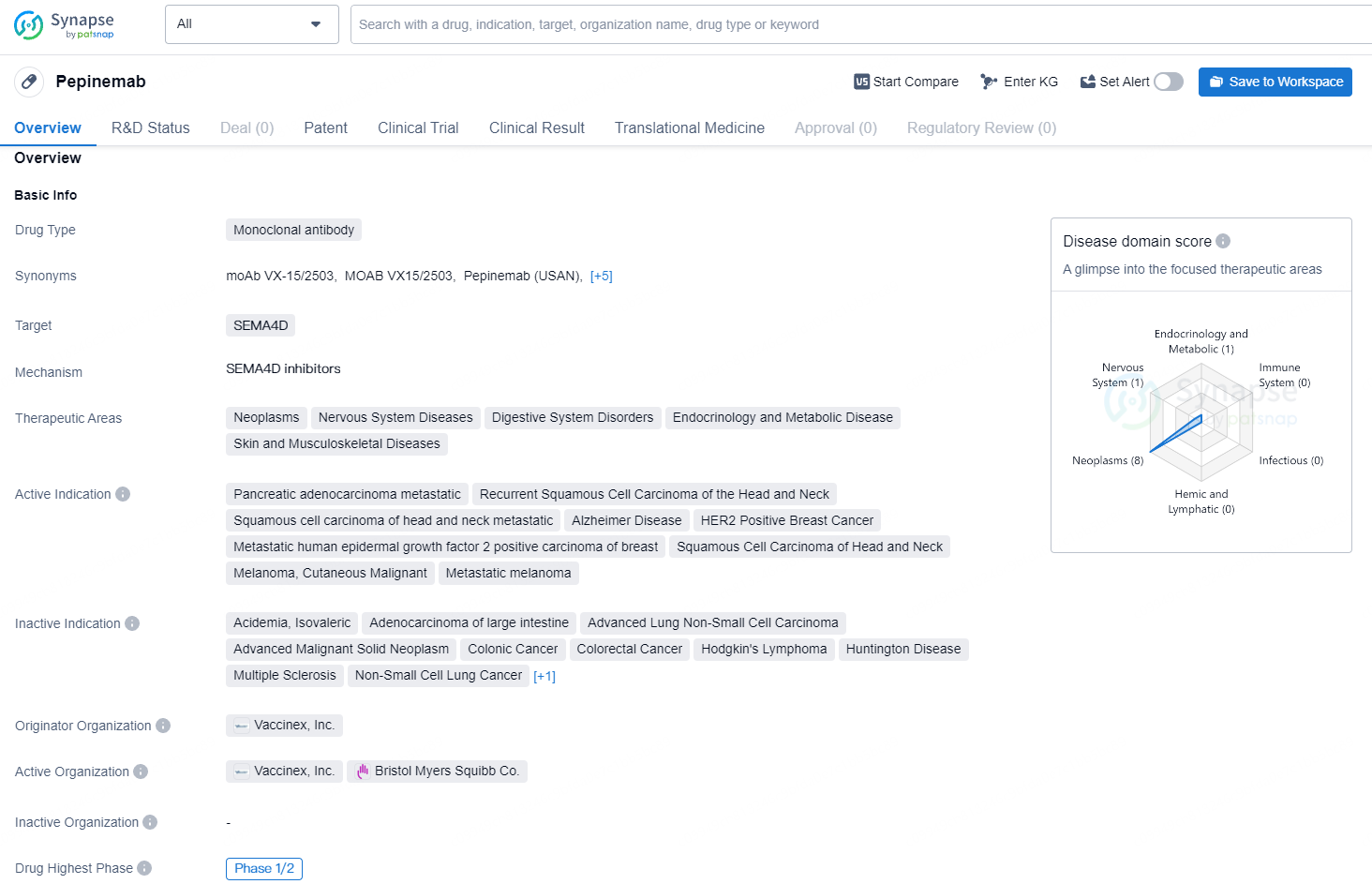
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The clinical trial successfully achieved its primary safety endpoint, demonstrating that pepinemab is well-tolerated in patients with Alzheimer's disease (AD). Across the 16 clinical sites involved, no serious treatment-emergent adverse events related to the medication were reported by the investigators. The only TEAE resulting in discontinuation throughout the trial occurred in the placebo group. Prior findings had shown that pepinemab was well-tolerated in patients with Huntington’s disease (HD) and multiple sclerosis (MS).
An essential secondary endpoint of this study was to evaluate whether pepinemab could prevent a decline in brain metabolic activity by blocking astrocyte reactivity. This was assessed via FDG-PET imaging signal increases in a critical brain region known to be impacted by disease progression over a 12-month treatment period with pepinemab compared to a placebo.
The medial temporal region of the brain, which includes the hippocampus and entorhinal cortex, is typically affected during the early stages of disease progression in many patients with mild cognitive impairment (MCI). Similar significant effects of pepinemab treatment on brain metabolic activity were previously observed in our phase 2 study of HD, suggesting mechanistic similarities in the pathology of these neurodegenerative diseases.
Although this current study was not powered sufficiently to detect cognitive effects or changes in additional secondary endpoints with statistical significance, previous studies involving approximately 90 HD patients per treatment arm with early cognitive symptoms akin to MCI in AD showed that pepinemab treatment enhanced performance on key cognitive and psychological assessments.
Pepinemab is a humanized IgG4 monoclonal antibody designed to inhibit SEMA4D, preventing its binding to plexin-B1 receptors, which otherwise induces the collapse of the actin cytoskeleton in cells and disrupts the homeostatic functions of astrocytes and other glial cells in the brain and dendritic cells in immune tissue. Pepinemab has shown a favorable safety profile and good tolerance across multiple clinical trials in various neurological and cancer indications.
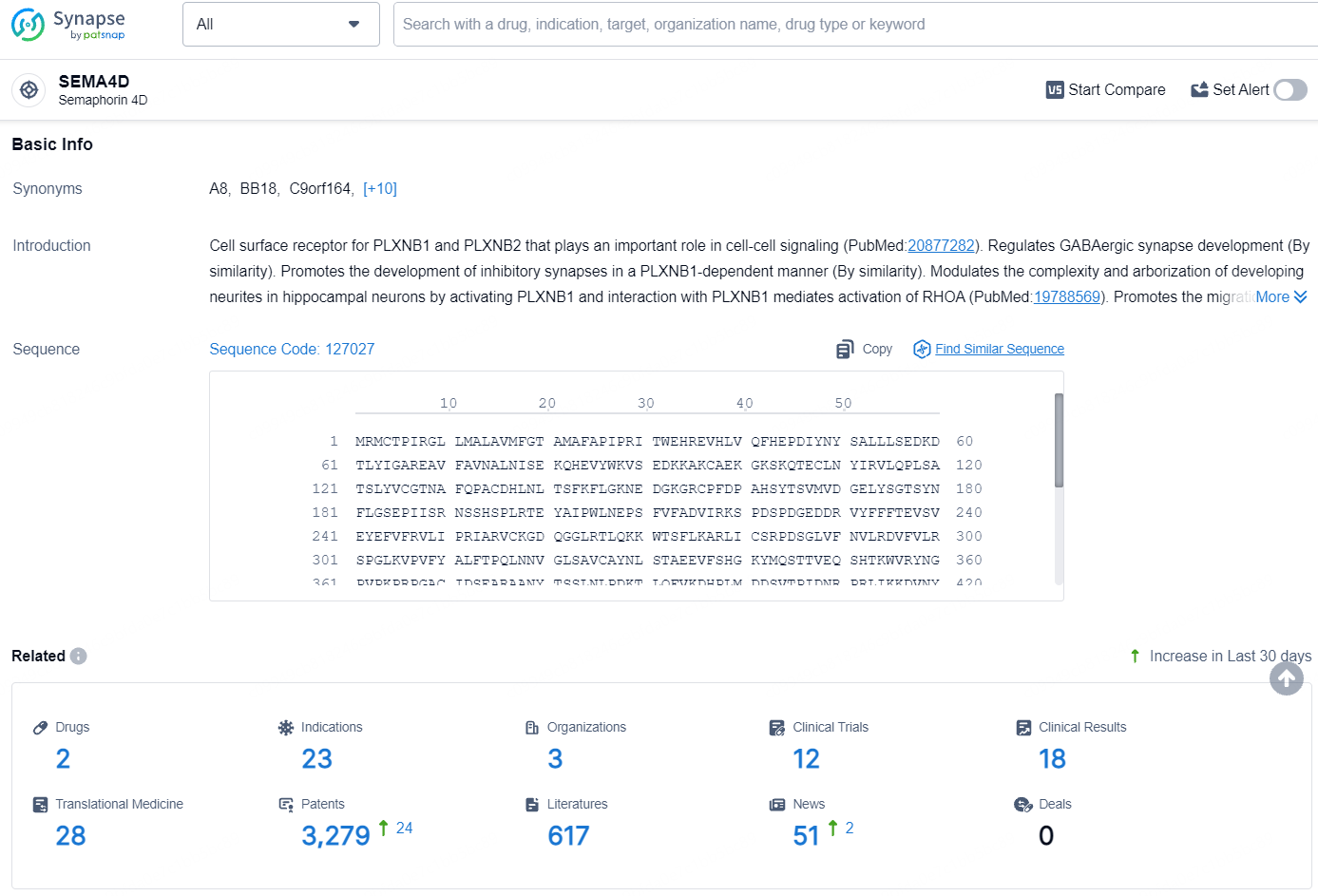
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 6, 2024, there are 2 investigational drugs for the SEMA4D targets, including 23 indications, 3 R&D institutions involved, with related clinical trials reaching 12, and as many as 3279 patents.
Pepinemab is a monoclonal antibody drug with a broad range of potential therapeutic applications, particularly in the treatment of advanced cancers and neurological disorders. With its targeting of SEMA4D, the drug has garnered significant interest in the pharmaceutical industry and has the potential to make significant contributions to the treatment of various diseases in the future.