In a Phase 2b trial, the oral peptide JNJ-2113 showed promise for moderate to severe psoriasis
Johnson & Johnson has publicized the results of its Phase 2b FRONTIER 1 study for JNJ-2113 in the renowned New England Journal of Medicine. Unique among potential treatments, JNJ-2113 is an orally administered peptide antagonist that specifically tackles the IL-23 receptor. This receptor is pivotal in driving the activation of harmful T-cells tied to moderate-to-severe plaque psoriasis. It also is fundamental to the inflammatory processes observed in PsO, as well as a range of other skin and gastrointestinal disorders that are influenced by IL-23.
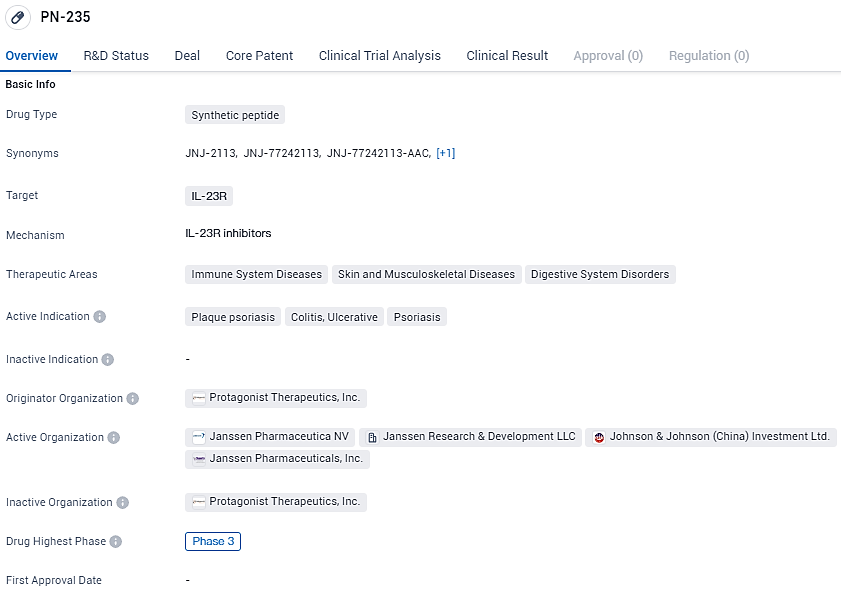
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The fundamental outcome measured in this research was a decline of 75% or more in the Psoriasis Area and Severity Index (PASI) score from the initial level by the 16th week. Data from the study indicated a notable dose-dependent effect for achieving PASI 75 by Week 16 in adult participants administered JNJ-2113 versus those given a placebo, with the top dosing regimen of 100 mg two times a day leading to 79% of individuals reaching a PASI 75 result.
"Recent years have seen remarkable progress in the understanding and treatment of immune-related inflammatory disorders such as Psoriasis (PsO). Patients are looking for treatments that maintain a balance between efficacy, tolerability, and ease of use," commented Robert Bissonnette, M.D., the CEO and Medical Director at Innovaderm Research based in Montreal, Canada.
Dr. Bissonnette added, "Data from the Phase 2b FRONTIER 1 trial, which has been published in the New England Journal of Medicine (NEJM), provide a very promising outlook for the future phases of clinical trials for JNJ-2113, highlighting its potential as an oral therapy that could be a convenient option for patients."
In conjunction with the use of JNJ-2113, patients experienced reductions in the serum concentrations of human beta-defensin 2 (hBD-2) when compared with those taking a placebo, with this effect noticeable from as early as the 4th week. The administration of 100 mg of the drug twice daily was linked to the lowest recorded hBD-2 levels starting from the 8th week. A clinical response associated with decreased levels of hBD-2 suggests that the IL-17/IL-23 pathway is being effectively targeted by the treatment.
The results from the FRONTIER 1 trial indicate that JNJ-2113 might have broader implications for treating various diseases involving IL-23. Subsequently, the company has commenced a Phase 2b ANTHEM-UC trial aiming to assess the efficacy and safety of JNJ-2113 in subjects with moderate to severe ulcerative colitis, comparing it to a placebo.
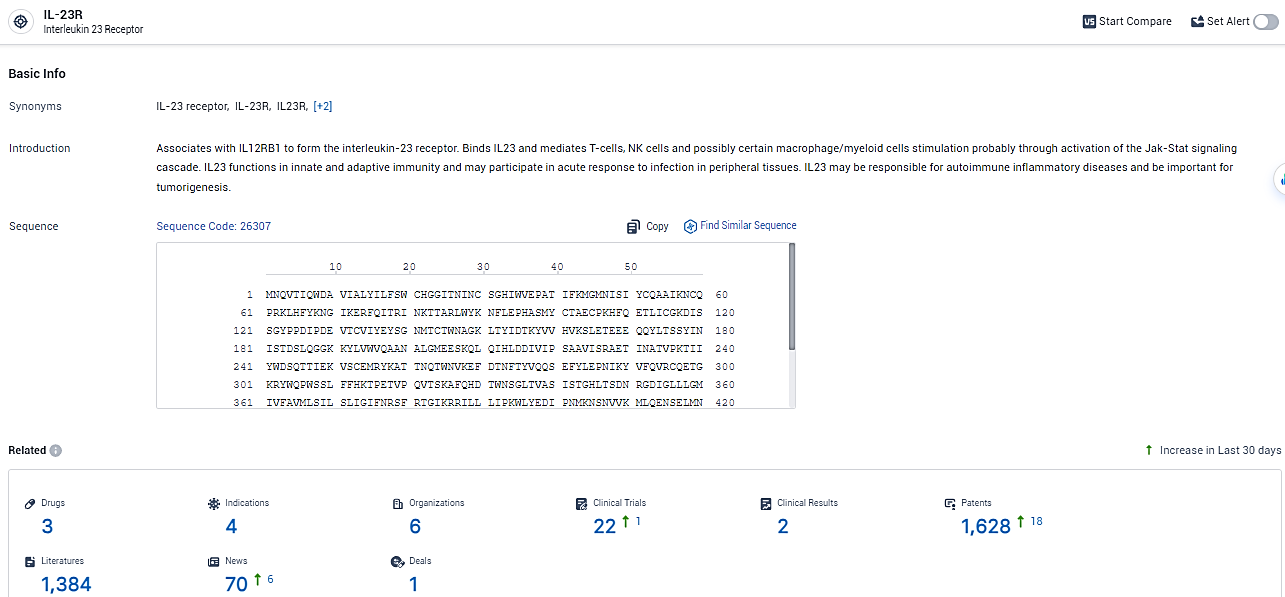
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 3 investigational drugs for the IL-23R target, including 4 indications, 6 R&D institutions involved, with related clinical trials reaching 22, and as many as 1628 patents.
JNJ-2113 targets IL-23R and is being investigated for its potential therapeutic benefits in immune system diseases, skin and musculoskeletal diseases, and digestive system disorders. With its highest phase of development being Phase 3, PN-235 has shown promise in earlier stages of testing and is now undergoing further evaluation in larger clinical trials.