Highlights of Financing and Strategic Collaborations in the Preclinical Biopharmaceutical Sector: February 2025(2)
Our analysis of February data reveals key trends in global preclinical-stage biopharmaceutical financing. For contextual understanding, we recommend beginning with the following article.
12.Harbour BioMed and Insilico Medicine Form Strategic Partnership to Advance AI-Driven Antibody Discovery and Development
Harbour BioMed and Insilico Medicine have established a strategic partnership aimed at accelerating the development of therapeutic antibodies by integrating artificial intelligence (AI) with antibody research. This collaboration seeks to leverage AI’s potential in antibody applications and target-specific research, aiming for breakthrough advancements in biopharmaceutical innovation. The partnership not only underscores AI’s growing importance in drug discovery but also highlights the transformative power of cross-industry collaboration in driving innovation.
According to the agreement, both companies will combine Harbour BioMed’s expertise in antibody development with Insilico Medicine’s AI-driven drug discovery platform. Notably, Insilico Medicine’s Integrated Drug Creation™ platform, which utilizes generative AI and a scalable wet lab system, can significantly reduce the time from data collection to molecular design and experimental validation to approximately six weeks. This highly efficient research process dramatically accelerates drug discovery and facilitates rapid clinical trial progression for promising drug candidates.
The initial phase of the collaboration will focus on specific disease targets, although financial terms and drug details have not been disclosed. However, given that similar partnerships in the industry often involve multimillion or even billion-dollar deals, both companies are expected to make substantial investments to ensure the project’s success. Additionally, Insilico Medicine has already achieved significant milestones in collaborations with major pharmaceutical companies, such as AstraZeneca, which suggests that its partnership with Harbour BioMed could generate substantial economic and social value.
As AI continues to reshape the healthcare industry, more pharmaceutical companies are exploring ways to integrate AI into their R&D pipelines. The Harbour BioMed-Insilico Medicine collaboration exemplifies this trend, demonstrating how AI-driven solutions can enhance global competitiveness and set a benchmark for industry innovation. Looking ahead, similar cross-sector collaborations are expected to emerge, further propelling advancements in biopharmaceutical research.
Despite its immense potential, AI-driven drug discovery still faces challenges, including data privacy concerns, algorithmic transparency issues, and regulatory uncertainties. While AI accelerates research efficiency, stakeholders must work collectively to address these challenges. Through continuous technological innovation and the establishment of robust regulatory frameworks, AI is poised to play an increasingly pivotal role in future drug discovery and development, ultimately providing more effective treatment options for patients worldwide.
13. Epitopea Partners with MSD to Develop RNA-Based Cancer Immunotherapies
On February 19, 2025, transatlantic cancer immunotherapy company Epitopea announced a licensing and research collaboration agreement with Merck & Co. (MSD) to identify Cryptigen™ tumor-specific antigens (TSAs) in an undisclosed solid tumor type. These antigens originate from non-coding regions previously considered "junk DNA" and exhibit shared, non-mutated, and aberrant expression across multiple patients. Through this collaboration, the two companies aim to leverage Epitopea's proprietary CryptoMap™ platform to discover novel immunogenic Cryptigen™ TSAs and develop off-the-shelf RNA-based immunotherapies for various refractory cancers.
Under the terms of the agreement, Epitopea will use its CryptoMap™ platform to identify and provide novel Cryptigen™ TSAs for predefined tumor types. MSD will have exclusive rights to develop and commercialize any therapeutic products resulting from the collaboration. In return, Epitopea will receive an undisclosed upfront payment and be eligible for milestone payments of up to $300 million per product. This financial arrangement not only highlights MSD’s commitment to the project but also underscores the immense potential of Epitopea’s technology platform.
At the core of Epitopea’s technology is its CryptoMap™ platform, which integrates immunopeptidomics, genomics, and bioinformatics pipelines to identify aberrantly expressed tumor-specific antigens hidden within cancer "junk" DNA. This innovative approach opens new avenues for developing highly effective immunotherapies targeting specific tumor types. Furthermore, since these antigens are commonly present across multiple patients, therapies based on them can be applied to a broader patient population, enhancing accessibility and efficacy.
This collaboration not only validates Epitopea’s technological capabilities but also positively impacts the entire cancer immunotherapy field. As more partnerships of this kind emerge, the number of therapies targeting newly discovered tumor-specific antigens is expected to grow significantly in the coming years. For MSD, this collaboration strengthens its leadership in immuno-oncology and could yield a series of breakthrough therapies. At the same time, it marks a step forward in the transition from traditional chemotherapy to more personalized and precise cancer treatments.
Since its founding, Epitopea has garnered support from top life sciences investors, including Advent Life Sciences, CTI Life Sciences, and Cambridge Innovation Capital. To date, the company has raised over $45 million, providing a strong financial foundation for its R&D activities. Epitopea plans to accelerate the development of its preclinical pipeline as it moves toward becoming a clinical-stage company. With the deepening collaboration with MSD, Epitopea is poised to introduce a range of innovative cancer immunotherapies in the coming years, offering hope to cancer patients worldwide.
14. Polymed Partners with Photys Therapeutics for Global Development of HPB-143
On February 18, 2025, Polymed announced a major partnership with Photys Therapeutics, granting Photys the rights to develop, manufacture, and commercialize the IRAK4-targeting protein degrader HPB-143 in all global markets except Greater China and Southeast Asia. In return, Polymed will receive multiple forms of compensation, including upfront and near-term payments, clinical development milestone payments, and sales royalties, while also holding an equity stake in Photys. This collaboration aims to accelerate the development of HPB-143 and expand its global market presence.
Compared to the IRAK4-targeting protein degrader KT-474, which is currently in Phase II clinical trials internationally, Polymed’s HPB-143 has demonstrated superior oral bioavailability, reaching 49% in mouse studies. Additionally, it exhibits weaker inhibition of the cardiac ion channel hERG, suggesting improved cardiovascular safety in humans. In multiple mouse disease models, HPB-143 has shown lower effective doses and superior efficacy. As a PROTAC (Proteolysis-Targeting Chimera), HPB-143 specifically degrades the IRAK4 protein by hijacking the intracellular ubiquitin-proteasome system, effectively modulating key signaling pathways involved in inflammatory responses and tumor progression.
IRAK4 plays a crucial role in human inflammatory responses and tumorigenesis, making it a highly promising target for protein degraders in treating autoimmune diseases such as atopic dermatitis and hidradenitis suppurativa. The therapeutic demand for these conditions remains largely unmet, with existing treatments often limited by efficacy or side effects. With its unique mechanism of action and superior pharmacological profile, HPB-143 has the potential to provide a safer and more effective treatment option for patients.
HPB-143 has already secured approval from both the U.S. FDA and China's National Medical Products Administration (NMPA) to conduct clinical trials. According to Photys Therapeutics' plan, a Phase I clinical study for HPB-143 is set to launch this year, marking a critical step in evaluating the drug's safety, tolerability, and preliminary efficacy. As clinical trials progress, HPB-143 has the potential to become one of the first-in-class IRAK4 degraders to reach the market, offering new hope for patients with autoimmune diseases worldwide.
This partnership not only reflects the global recognition of Polymed’s PROTAC drug development platform but also signifies the commitment of both companies to advancing HPB-143’s development through resource integration. Photys Therapeutics, leveraging its proprietary PHICS™ technology and other proximity-based modalities, is dedicated to developing a pipeline of therapies targeting cancer, autoimmune diseases, and cardiometabolic disorders. Through this collaboration, both companies have the opportunity to expand their product portfolios and achieve multiple key development milestones in the coming years. For the broader pharmaceutical industry, this partnership serves as a positive signal of the potential of innovative therapies in addressing unmet medical needs.
15. Perceive Pharma Secures $15 Million Series A Funding to Advance Ophthalmic Neuroprotective Therapy
On February 18, 2025, Perceive Pharma, a company focused on developing novel small-molecule ophthalmic therapeutics, announced the completion of a $15 million Series A funding round. The financing was backed by several renowned investment firms, including Deerfield Management, Johnson & Johnson Innovation – JJDC, Inc., Braidwell LP, GV, Retinal Degeneration Fund, and Catalio Capital Management, LP. The funds will be used to advance its glaucoma program to the clinical readiness stage and further expand its research pipeline.
One of Perceive Pharma’s core products is PBI-671, a first-in-class drug candidate designed to prevent vision loss caused by glaucoma. All currently approved glaucoma treatments focus on reducing intraocular pressure (IOP), yet approximately half of glaucoma patients continue to experience vision loss despite having normal IOP. PBI-671 is based on a neuroprotective pathway first identified through genomic screening and aims to directly protect retinal cells, offering a potential treatment for patients whose disease continues to progress despite existing therapies.
Perceive Pharma's research and development efforts are built on a strong scientific foundation, providing the company with a unique advantage in identifying new therapeutic targets and developing transformative vision-protection therapies. With the successful completion of its Series A financing, the company will accelerate the advancement of its lead therapy to address the medical needs of more than 1.5 million patients worldwide suffering from progressive glaucoma. Additionally, Perceive Pharma plans to explore treatment opportunities for other undisclosed diseases.
Notably, Perceive Pharma is part of JLABS @ San Diego, a division of Johnson & Johnson Innovation – JLABS, a global life sciences incubator network. Through this platform, startups gain access to cost-efficient laboratory space, equipment resources, and expert support, helping to accelerate the development of innovative products. Such support is crucial for emerging companies like Perceive Pharma as they navigate critical growth and development stages.
16. Lexenpharm and Sun-Novo Pharmaceutical Join Forces to Revolutionize Asthma and COPD Treatment
Recently, Lexenpharm and Sun-Novo Pharmaceutical announced a regional strategic partnership for the development of two innovative drug projects, LXP2311 and LXP0531, in mainland China. Through this collaboration, both companies will leverage their technological expertise to advance the research, development, and commercialization of these breakthrough therapies, bringing new hope to the medical industry.
Current COPD treatments primarily rely on short-acting or long-acting monotherapies, which often have limited efficacy and duration of action. To address this challenge, Lexenpharm and Sun-Novo have jointly developed LXP2311 and LXP0531, the world's first ultra-long-acting combination inhalation formulations. In particular, LXP2311, the first ultra-long-acting LABA/LAMA combination therapy, requires only once-daily administration and can be delivered via a standard jet nebulizer, significantly enhancing patient convenience. Meanwhile, LXP0531 is the first ultra-long-acting ICS/LABA combination therapy, recommended for the treatment of asthma and COPD, offering improved ease of use for both physicians and patients.
The success of the LXP2311 and LXP0531 projects is supported by five core technologies, including nebulized inhalation formulation and soft mist inhalation technology. These advancements not only enhance drug efficacy and safety but also significantly improve the patient experience. For instance, the application of nano-scale sterile suspension technology and Blow-Fill-Seal (BFS) aseptic technology ensures efficient drug delivery and stability within the body, providing better disease control. These technological innovations are expected to have a substantial impact on improving treatment outcomes for asthma and COPD patients worldwide.
Asthma and COPD remain major global public health concerns, with COPD ranking as the second leading disease in China and the third leading cause of death worldwide. It is estimated that COPD results in an annual global economic burden exceeding $4.3 trillion, with China alone bearing a financial burden of $1.36 trillion. Given the severity of this situation, the development of more effective and convenient treatment options is an urgent necessity. The partnership between Lexenpharm and Sun-Novo reflects their commitment to addressing this critical need by providing better therapeutic solutions for patients.
17.DAAN Biotherapeutics and LigaChem Biosciences Enter Exclusive Antibody Licensing Agreement to Advance ADC Therapy Development
February 13, 2025, Seoul, South Korea – DAAN Biotherapeutics, a leading innovative drug development company specializing in T-cell receptor (TCR)-based therapies, has announced an exclusive licensing agreement with LigaChem Biosciences. This collaboration aims to develop a novel tumor-targeting antibody to facilitate the advancement and commercialization of antibody-drug conjugates (ADCs). By leveraging their respective technological strengths, the two companies will work together to drive progress in ADC therapies for solid tumor patients.
With its cutting-edge next-generation antibody technology, superior target discovery, and antibody development capabilities, DAAN Biotherapeutics has successfully built a proprietary pipeline focused on developing antibodies targeting tumor markers overexpressed in solid cancers. This achievement is supported by its strategic partnership with OmniAb. Additionally, DAAN has developed a platform called TACTIC (Tumor-Activated Conditional T-cell Connector), designed to be a global leader in solid tumor-targeted T-cell activators. These technological advancements provide a strong foundation for the development of ADC therapies.
The collaboration between DAAN Biotherapeutics and LigaChem Biosciences represents an effective integration of their technologies and resources, marking a significant milestone in the field of solid tumor treatment. It is expected to accelerate the development of novel ADC therapies and provide patients with more effective treatment options. As the partnership deepens, more research findings are anticipated to translate into practical applications, contributing to the fight against cancer.
Looking ahead, DAAN Biotherapeutics plans to continue expanding its collaborations with global pharmaceutical companies, particularly in the ADC sector. Furthermore, the company aims to achieve breakthroughs in T-cell therapies, especially through the development of novel T-cell connectors using its TACTIC platform. Through continuous exploration and innovation, DAAN Biotherapeutics is steadily advancing toward its goal of becoming a global leader in cancer treatment.
18.Xilio Therapeutics Announces Multiple Masked T-cell Engager Programs and Collaboration Agreement with AbbVie
February 12, 2025 – Xilio Therapeutics, Inc., a clinical-stage biotechnology company focused on discovering and developing tumor-activated immunotherapies, has announced three wholly-owned preclinical programs targeting prostate-specific membrane antigen (PSMA), claudin 18.2 (CLDN18.2), and six-transmembrane epithelial antigen of the prostate 1 (STEAP1) with masked T-cell engagers. Additionally, Xilio has entered into a collaboration, licensing, and option agreement with AbbVie to leverage Xilio’s proprietary tumor-activated technology platform to develop novel tumor-activated immunotherapies.
T-cell engagers are designed to direct immune effector cells against cancer cells by simultaneously binding tumor-associated antigens expressed on the surface of cancer cells and the T-cell receptor complex on T cells, thereby inducing T-cell-mediated tumor cell killing. However, despite their significant therapeutic potential in various advanced solid tumors, the widespread application of T-cell engagers has been limited by toxicity concerns. Xilio is utilizing its clinically validated tumor-activated platform to advance a series of novel masked T-cell engagers with conditionally regulated half-life properties, aiming to achieve potent, localized T-cell activation and tumor cell destruction while improving the therapeutic index.
Xilio is currently progressing three wholly-owned preclinical programs, each targeting a distinct tumor-associated antigen:
·PSMA – a promising T-cell engager target for prostate cancer
·CLDN18.2 – a potential T-cell engager target for gastric, pancreatic, esophageal, and lung cancers
·STEAP1 – a viable target for prostate, colorectal, and lung cancers
Xilio expects to nominate development candidates and submit Investigational New Drug (IND) applications for these programs in the coming years.
Under the terms of the collaboration agreement with AbbVie, Xilio will receive a total upfront payment of $52 million, including $42 million in cash and $10 million in Xilio common stock purchased by AbbVie at a premium. Xilio has granted AbbVie an exclusive option to initiate an initial program for the discovery and development of masked T-cell engagers, with the potential for two additional programs to be launched under the agreement. Moreover, Xilio will be eligible for up to $2.1 billion in additional milestone payments, including option fees, as well as development, regulatory, and commercial sales milestones.
Based on its current operational plans, Xilio expects its existing cash and equivalents, combined with the upfront payment from the AbbVie agreement, to fund its operating and capital expenditure needs until the first quarter of 2026. Xilio will hold an investor conference call and webcast on February 12, 2025, to provide further details on this announcement and invite investor participation. These strategic moves not only strengthen Xilio’s financial position but also ensure continued investment in future research and development.
19.Ellipses Pharma Acquires First-in-Class Next-Generation Immuno-Oncology Drug GENA-104 to Accelerate Cancer Treatment Development
Ellipses Pharma Limited (Ellipses), a global drug development company dedicated to accelerating cancer treatment through innovative drug development models, announced on February 11, 2025, that it will develop a next-generation immuno-oncology drug aimed at addressing the needs of cancer patients who do not respond to existing checkpoint inhibitors. Ellipses has secured the global rights to ‘GENA-104,’ a first-in-class immuno-oncology monoclonal antibody targeting CNTN4, a recently identified checkpoint protein highly expressed across multiple tumor types.
GENA-104 was originally discovered by the South Korea-based biotechnology company Genome & Company (Genome), which specializes in the development of novel cancer-targeting antibodies and antibody-drug conjugates (ADCs). Under the agreement with Genome, Ellipses will assume full responsibility for the future clinical development of GENA-104, designating it as EP0089. The potential of EP0089 as a novel immuno-oncology therapy was highlighted in a landmark study published in Science Immunology in October 2024.
The Phase I IND application for EP0089 was approved by the South Korean Ministry of Food and Drug Safety in January 2024. Ellipses plans to initiate Phase I clinical trials in South Korea in 2025 and subsequently expand to the United States and Europe upon obtaining the necessary regulatory approvals. This new therapeutic approach aims to inhibit the CNTN4-APP checkpoint interaction on T cells, thereby enhancing tumor cell destruction, making it particularly promising for cancers resistant to traditional checkpoint inhibitors.
This collaboration marks a significant milestone for Ellipses Pharma in expanding its pipeline and represents a crucial advancement in cancer treatment. By acquiring breakthrough candidates such as EP0089, Ellipses reaffirms its commitment to rapidly identifying and developing top-tier therapies to provide new treatment options for patients as quickly as possible. As further research data emerges and clinical trials progress, EP0089 has the potential to transform the treatment landscape for certain refractory cancers, offering new hope to patients worldwide.
20.Alloy Therapeutics Partners with Pfizer to Develop a New Antibody Discovery Platform
Boston, February 11, 2025 – Alloy Therapeutics Inc. (Alloy), a biotechnology ecosystem company committed to democratizing access to cutting-edge drug discovery technologies, has announced its latest strategic collaboration with Pfizer. This partnership aims to develop a novel platform designed to enhance Pfizer’s ability to discover highly specific and effective antibodies for challenging targets that current antibody discovery technologies struggle to address. Under the terms of the agreement, Alloy will receive an upfront payment from Pfizer and will be eligible for pre-specified preclinical-to-commercial milestone payments related to products developed using the new platform.
Alloy’s ATX-Gx platform has rapidly become the industry standard for fully humanized transgenic mice, utilized by over 170 partners to advance various therapeutic discovery programs. Alloy remains committed to reinvesting its revenue into innovation and has continuously expanded its murine platform by incorporating ATX-GL™, which features a complete human lambda library, and ATX-GKH™, an ultra-immune strain designed to enhance antigen-specific B-cell production and improve IgG class switching.
In addition to this collaboration, Alloy and Pfizer have worked together on multiple antibody discovery initiatives, including the use of Alloy’s ATX-CLC platform. This platform enables the expression of common light-chain antibodies with full heavy-chain diversity, facilitating the efficient and modular discovery of bispecific antibodies.
21.Bolt Biotherapeutics and Toray Collaborate on a Novel ISAC Therapy Targeting Caprin-1
On February 11, 2025, Bolt Biotherapeutics announced a global collaboration with Toray Industries to co-develop a therapeutic targeting Caprin-1, a novel cancer target discovered by Toray. The two companies are working together to develop a Boltbody™ immune-stimulating antibody-drug conjugate (ISAC) targeting Caprin-1, intended for use in multiple solid tumor types. Under the existing joint development and licensing agreement, Toray is providing its proprietary Caprin-1-targeting antibody, TRK-950, while Bolt contributes its proprietary linker-payload technology from the Boltbody™ ISAC platform.
Caprin-1 is a tumor-specific antigen that is highly expressed on the cell membrane of most solid tumors while exhibiting minimal expression in normal tissues. Additionally, Caprin-1 has been shown to promote tumor growth and metastasis. Toray’s asset, TRK-950, is a monoclonal antibody targeting Caprin-1 and is currently in Phase II development for gastric cancer, validating the antigen’s potential as an ISAC target. TRK-950 functions by binding to and attacking cancer cells expressing Caprin-1, with particularly strong expression observed in gastric cancer and other solid tumors, as well as in metastatic cancer cells and cancer stem cells.
Bolt’s Boltbody™ ISAC platform combines the precision of antibodies with the strength of both innate and adaptive immune systems to reprogram the tumor microenvironment and generate potent anti-cancer responses. Each Boltbody™ ISAC candidate comprises a tumor-targeting antibody, a non-cleavable linker, and a proprietary immune-stimulatory payload. This combination activates myeloid cells, initiating a positive feedback loop that releases cytokines and chemokines, attracts additional immune cells, lowers the activation threshold of immune responses, and enhances the number of activated immune system cells in the tumor microenvironment, ultimately promoting a robust immune response.
In recent years, Toray has increasingly focused on "Life Innovation," accelerating advancements in medical technology and disease prevention with the goal of alleviating the burden on healthcare professionals and helping people live longer, healthier lives. Oncology has been identified as a key area of focus, and Toray is committed to developing industry-leading therapeutic approaches with the potential to significantly improve patient outcomes. The collaboration with Bolt is part of Toray’s broader mission to create a healthier society through the development of innovative medicines, reflecting the shared commitment of both companies to providing better treatment options for cancer patients across various indications.
This partnership builds upon the highly productive interactions between Alloy and Pfizer, further solidifying the companies’ collaboration in antibody discovery and technological innovation. Through joint efforts, the two parties aim to drive scientific progress and accelerate the development of new therapies, ultimately benefiting patients. As the collaboration advances, it is expected to yield additional breakthrough innovations that will contribute to the global healthcare landscape.
22.XtalPi Holdings and Probable Biotechnology Join Forces to Accelerate First-in-Class Drug Discovery
On February 10, 2025, XtalPi Holdings announced the completion of a strategic investment in Probable Biotechnology and the establishment of an in-depth research and development collaboration. Together, the two companies will advance the discovery and development of First-in-Class (FIC) drugs targeting novel mechanisms identified by Probable Biotechnology to address significant unmet clinical needs. This partnership aims to leverage XtalPi’s cutting-edge AI and robotics-driven platform alongside Probable Biotechnology’s pioneering research in translational regulation to accelerate drug development.
Probable Biotechnology specializes in modulating cellular states through ribosome and kinase methylation switches, developing small-molecule targeted therapies for major diseases such as cancer and inflammation. XtalPi, known for its industry-leading AI and robotics-powered drug discovery platform, integrates high-precision quantum physics-based computational chemistry algorithms with over 300 AI models. This platform enables efficient exploration of large-scale chemical space to design and optimize molecules with both innovation and strong drug-like properties. By partnering with XtalPi, Probable Biotechnology will gain access to powerful computational and experimental tools, significantly enhancing the speed and success rate of its FIC drug development efforts.
The first project under this collaboration focuses on small-molecule inhibitors targeting the RAS-MAPK signaling pathway, particularly for pan-cancer indications associated with RAS mutations. These inhibitors have demonstrated the ability to overcome resistance to KRAS-targeted therapies in resistant mouse models. When used in combination with existing standard-of-care therapies, they have shown significant advantages in tumor growth inhibition, prolonged survival, and reduced mortality in preclinical studies.
Additionally, Probable Biotechnology is developing a novel small-molecule inhibitor for the treatment of acute pancreatitis. Preclinical data indicate promising pharmacokinetic properties, efficacy, and a favorable safety profile, offering a potential new therapeutic option for patients.
By combining Probable Biotechnology’s expertise in translational regulation with XtalPi’s AI-driven drug discovery capabilities, this partnership aims to push the boundaries of innovation in drug development. Through this collaboration, the two companies seek to accelerate the discovery of transformative therapies, bringing hope to patients suffering from challenging diseases worldwide.
23.LEO Pharma and DEBRA Research Collaborate to Advance Treatment for Epidermolysis Bullosa
LEO Pharma and DEBRA Research announced a partnership aimed at accelerating the development of treatments for epidermolysis bullosa (EB). This rare genetic disorder causes extremely fragile skin that can blister and develop wounds from minor friction or trauma. The collaboration will not only enhance understanding of EB's pathological mechanisms but also develop new therapeutic strategies to improve patients' quality of life.
Epidermolysis bullosa is a group of heterogeneous genetic disorders characterized by mutations in structural skin proteins that lead to varying degrees of skin and mucosal fragility. Current treatments primarily focus on wound care, pain management, and infection prevention, lacking fundamental therapies targeting the underlying cause. For certain severe types of EB, such as dystrophic EB, existing treatments often fail to effectively control symptoms, creating an urgent need for innovative therapeutic approaches.
The collaboration between LEO Pharma and DEBRA Research will focus on basic research and clinical trials, exploring various novel therapies including gene therapy. For example, the topical gene therapy B-VEC has shown significant efficacy in promoting wound healing in dystrophic EB patients during Phase 3 clinical trials. Additionally, researchers are investigating other cell-based therapies such as bone marrow transplantation and protein replacement therapy. Through this partnership, both parties hope to discover more effective treatments and accelerate their translation into clinical applications.
While the specific financial terms of the collaboration remain undisclosed, it is anticipated that LEO Pharma, as an international pharmaceutical company specializing in dermatology, will provide substantial funding and technical resources for the project. This will help accelerate the translation from laboratory to market, enabling new therapies to benefit patients more quickly. Meanwhile, DEBRA Research, as an organization long committed to EB research and patient support, will contribute its disease-specific expertise and network resources.
As the collaboration between LEO Pharma and DEBRA Research deepens, it is expected to yield a series of breakthrough research outcomes in the near future. Beyond directly improving the health of EB patients, this partnership will also enhance the medical community's understanding of genetic skin disorders. More importantly, it demonstrates the tremendous potential of cross-institutional collaboration in addressing rare diseases, providing a replicable model for treating other similar conditions. Ultimately, such collaborations may alleviate the socioeconomic burden on EB patients and their families while improving their quality of life.
Summary
Looking back at February 2025, we can observe several notable characteristics and trends in financing, collaborations, and licensing activities in the preclinical drug sector. First, capital market enthusiasm has not waned; rather, driven by both policy support and technological advancements, investment in the biopharmaceutical industry has continued to grow. According to statistics, by the end of February, the global biopharmaceutical sector had completed financing activities totaling over $3.2 billion, providing crucial funding support to numerous early-stage R&D companies and helping them bridge the gap from laboratory to market.
Second, in terms of international collaborations, Chinese companies are gradually becoming a force to be reckoned with. Whether through technology import or product export, Chinese pharmaceutical firms are actively exploring partnership models with international counterparts to accelerate their development. This is particularly evident in the antibody-drug conjugate (ADC) field, where Chinese companies have performed exceptionally well. A prime example is the collaboration between CSPC Pharmaceutical and Radiance Biopharma. Such cross-border partnerships not only enhance product market competitiveness but also lay a solid foundation for subsequent R&D efforts.
How to get the latest progress on drug deals?
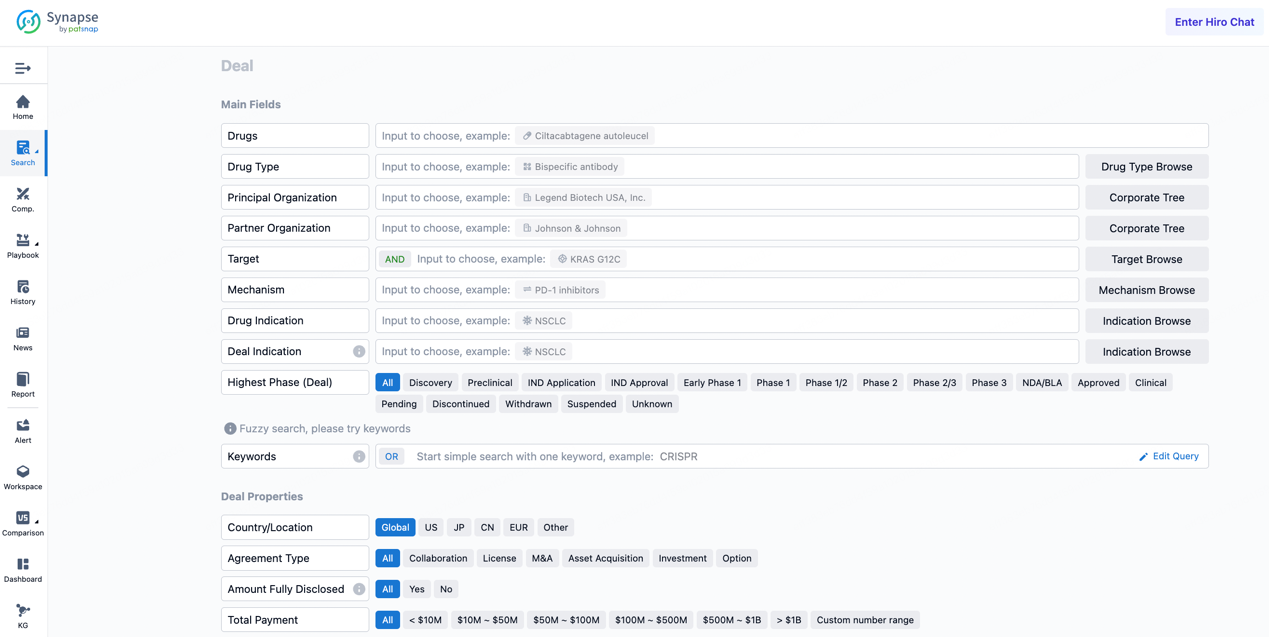
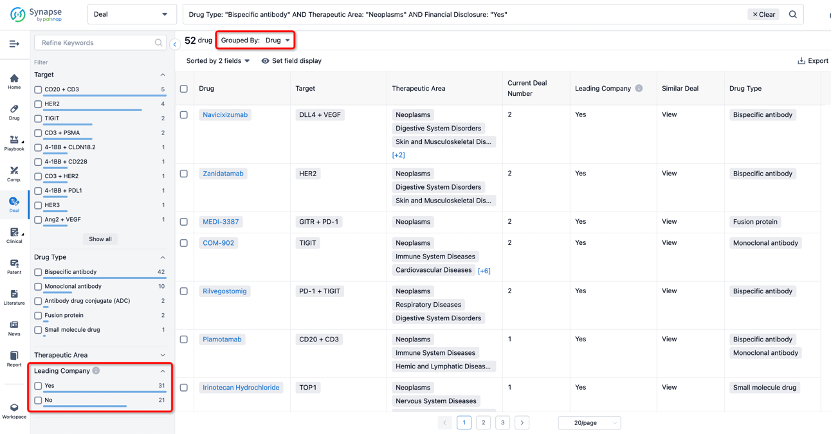
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
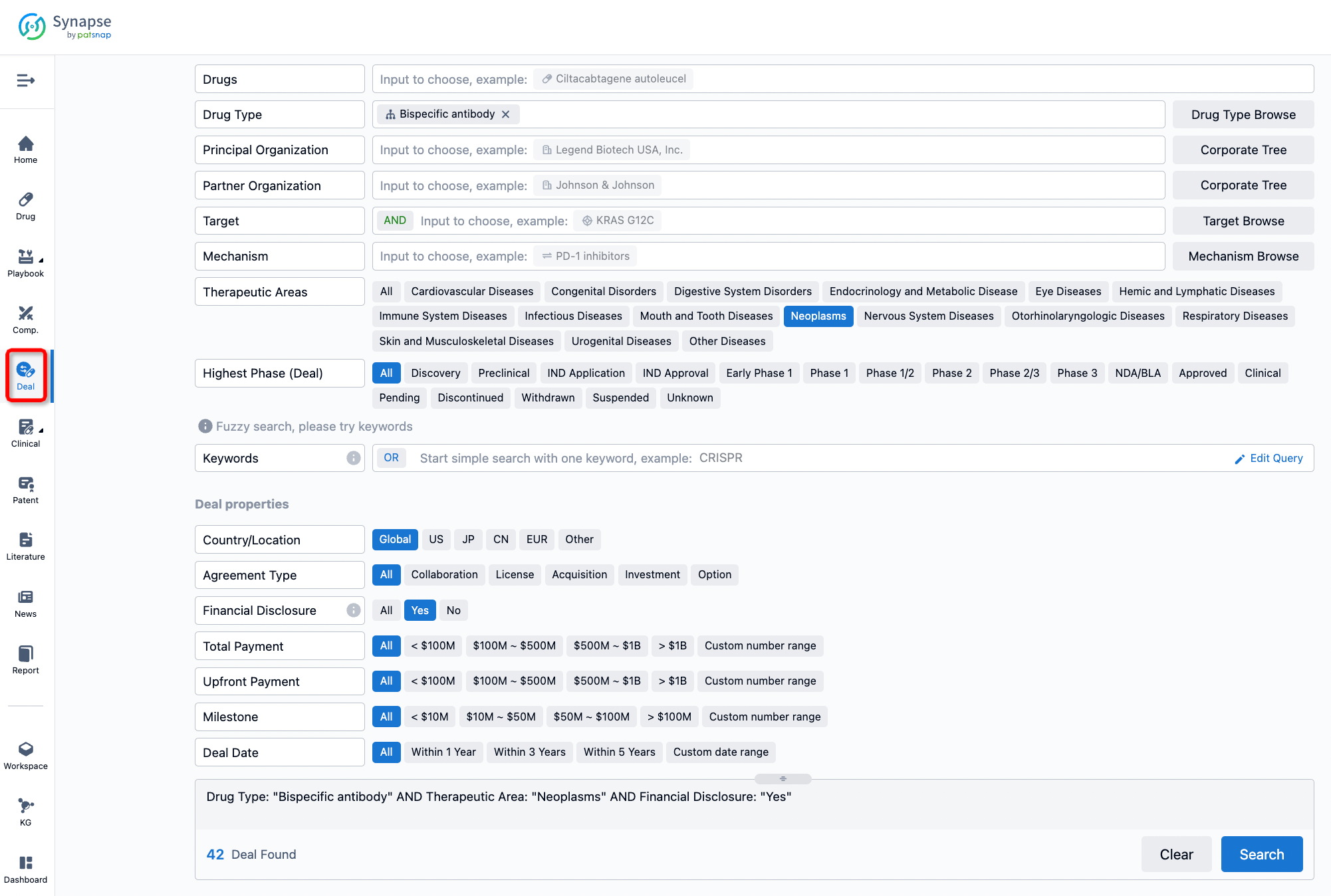
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
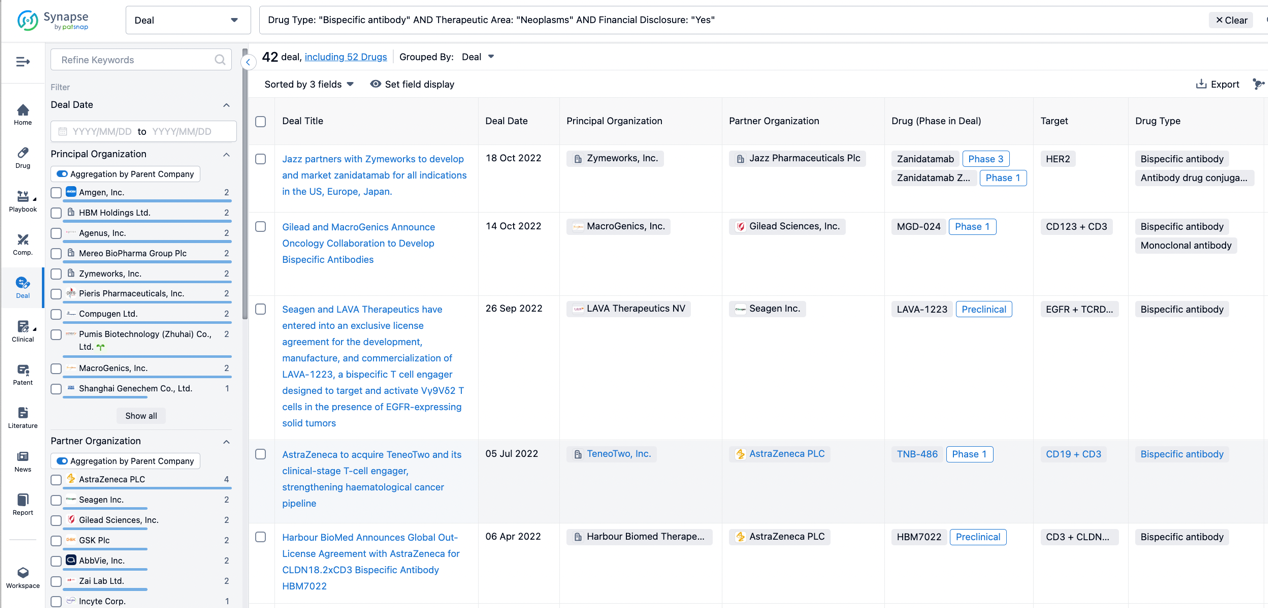
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
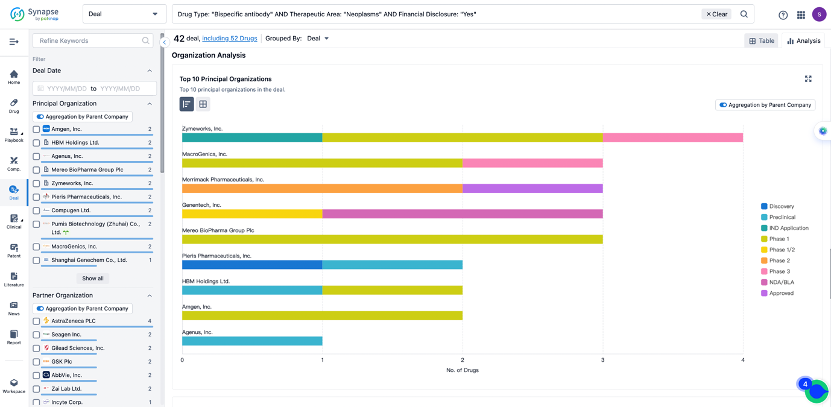
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
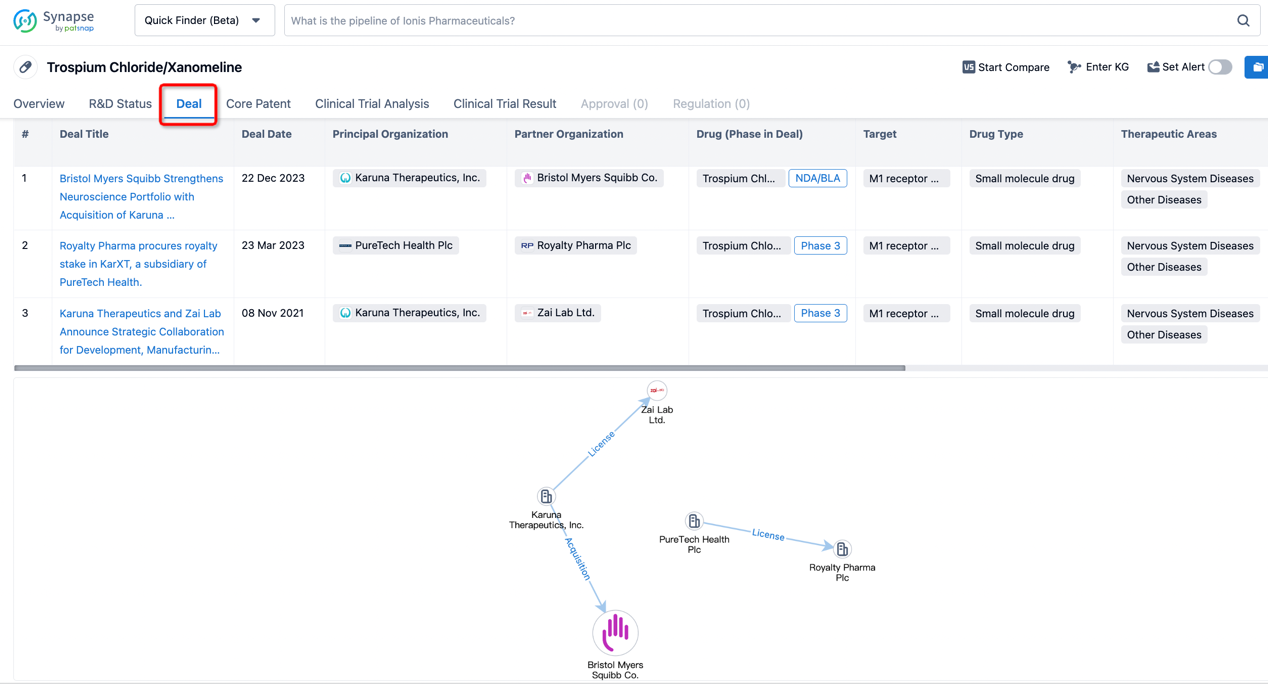
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
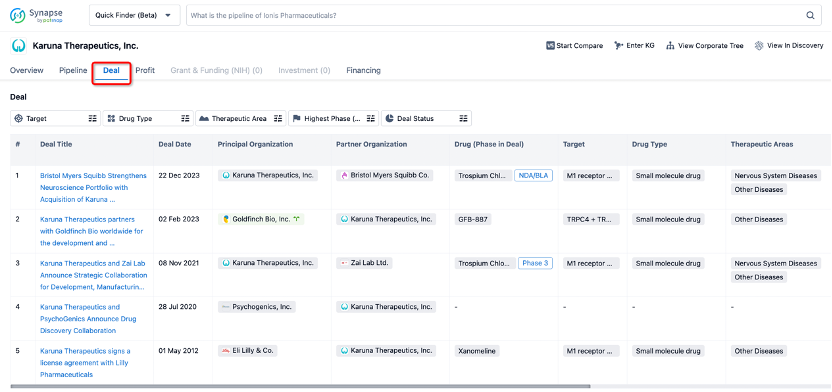
Click on the image below to explore new pharmaceutical funding transactions!