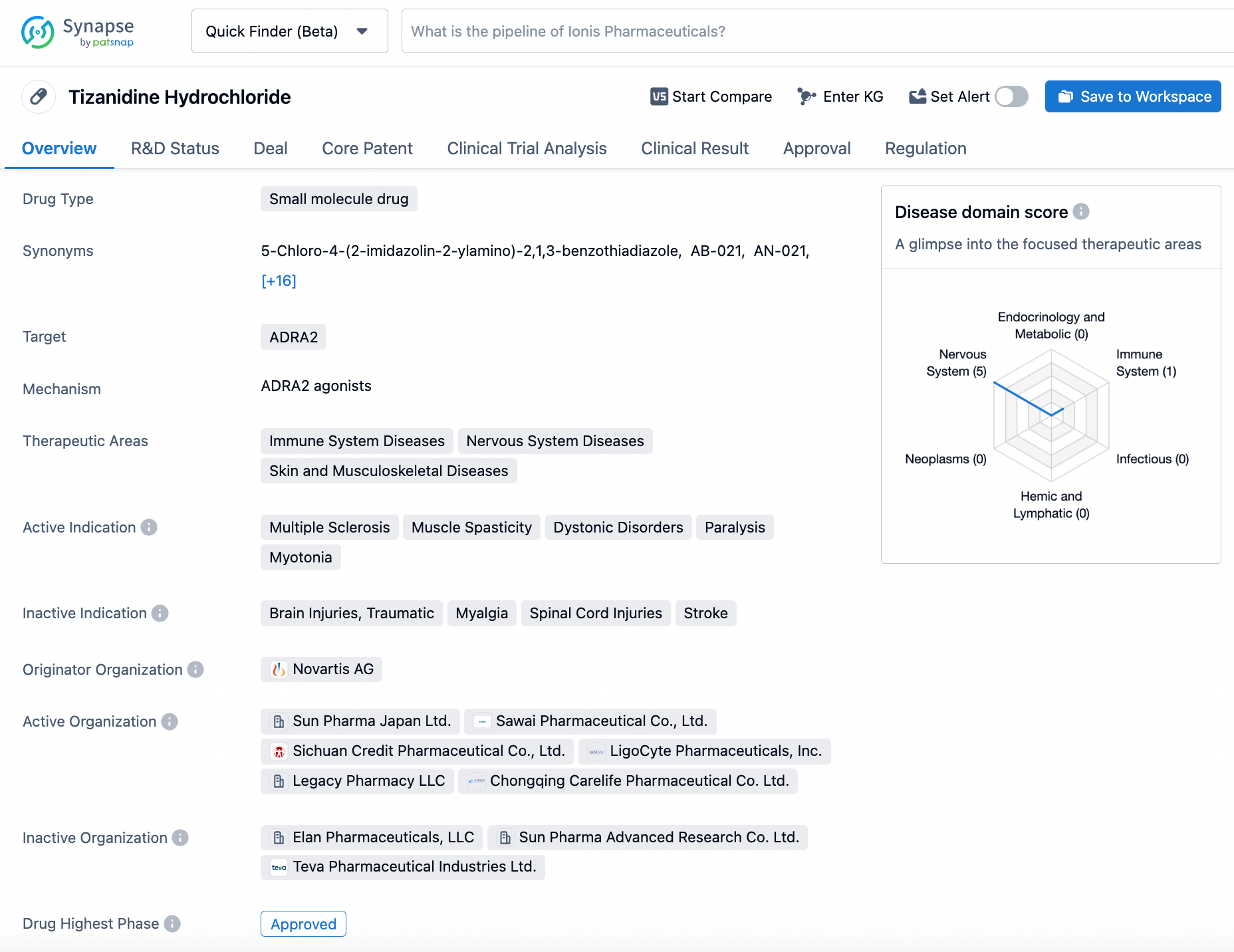
How to Effectively Search for Tizanidine on Synapse
Tizanidine Hydrochloride, a minuscule molecule drug, is a potent agonist that targets the ADRA2 receptor with high specificity. This drug is extensively employed in the management of muscle spasticity in patients with multiple sclerosis, paralysis, and myotonia. It was granted the first approval on January 20th, 1988, and was developed by the esteemed pharmaceutical company, Novartis. Tizanidine Hydrochloride acts as an agonist to the ADRA2 receptor within the central nervous system, resulting in a marked decrease in the release of excitatory neurotransmitters, which efficiently reduces muscle spasticity. The drug, however, should be used with caution as it can cause certain side effects, including drowsiness, dizziness, and dry mouth. Additionally, taking Tizanidine Hydrochloride with other medications that can cause sedation is not recommended. To ensure its safe and effective use, Tizanidine Hydrochloride should only be used under the close supervision of a healthcare provider, and patients should be carefully monitored for any adverse effects. Click on the image below to begin the exploration journey of Tizanidine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!