How to find the sequence of Pegaptanib?
Pegaptanib, developed by EyeTech Pharmaceuticals and later acquired by OSI Pharmaceuticals, is a pegylated aptamer that targets vascular endothelial growth factor (VEGF). VEGF is a signaling protein that plays a crucial role in angiogenesis, the formation of new blood vessels, which is a key process in the development and progression of various diseases, including age-related macular degeneration (AMD). Pegaptanib is indicated for the treatment of neovascular (wet) AMD, a condition characterized by the abnormal growth of blood vessels under the retina, leading to vision loss. By targeting VEGF, pegaptanib aims to inhibit the formation and leakage of these abnormal blood vessels, thereby preserving and potentially improving visual acuity.
Summary of Research Progress of Pegaptanib
Pegaptanib works by binding to the VEGF165 isoform, the most abundant form of VEGF in the eye, with high affinity and specificity. This binding prevents VEGF from interacting with its receptors on the surface of endothelial cells, thus inhibiting the signaling pathways that promote angiogenesis and vascular permeability. By blocking VEGF activity, pegaptanib reduces the formation and leakage of abnormal blood vessels, helping to stabilize and improve vision in patients with wet AMD. Pegaptanib is administered via intravitreal injection, which allows for direct delivery to the affected area of the eye. The drug was approved by the U.S. Food and Drug Administration (FDA) in 2004 and has since been used in clinical practice, although its usage has declined with the introduction of newer and more effective therapies.
Globally, the competition in the wet AMD market is robust, with several companies developing and marketing advanced therapies. Pegaptanib faces competition from anti-VEGF drugs such as ranibizumab (Lucentis) and aflibercept (Eylea), which are more widely used due to their superior efficacy and longer duration of action. Ranibizumab and aflibercept are both recombinant monoclonal antibodies that target all isoforms of VEGF, providing broader and more potent inhibition. Additionally, newer therapies like brolucizumab (Beovu) and faricimab (Vabysmo) have entered the market, offering additional options for patients. Despite this competition, pegaptanib remains a significant player in the history of wet AMD treatment and has contributed to the understanding of the role of VEGF in the disease.
Clinical research on pegaptanib has demonstrated its efficacy and safety in treating wet AMD. The Phase 3 clinical trials, such as the VISION study, showed that pegaptanib could significantly reduce the risk of vision loss and improve visual acuity in patients with wet AMD. The drug was generally well-tolerated, with common side effects including conjunctival hemorrhage, eye pain, and increased intraocular pressure. Ongoing and future trials aim to further evaluate the long-term safety and efficacy of pegaptanib, as well as explore its potential in combination with other therapies to achieve better outcomes. While newer therapies have surpassed pegaptanib in terms of efficacy, the drug's historical significance and contributions to the field of wet AMD treatment remain noteworthy.
Sequence Characteristics of Pegaptanib
Pegaptanib is a 28-nucleotide RNA-based aptamer that has been modified with polyethylene glycol (PEG) to enhance its stability and pharmacokinetic properties. The sequence of pegaptanib is specifically designed to bind to the VEGF165 isoform with high affinity and specificity. This sequence is optimized to ensure that the aptamer can effectively recognize and bind to the target VEGF165, which is crucial for the drug's therapeutic efficacy and safety. The precise sequence of pegaptanib is a proprietary information, but it is known to be highly complementary to the VEGF165 isoform, allowing for effective inhibition of VEGF activity.
Chemical Modification and Species of pegaptanib
The chemical modifications in pegaptanib include the addition of polyethylene glycol (PEG) moieties. PEGylation involves the covalent attachment of PEG chains to the aptamer, which enhances the stability and pharmacokinetic properties of the drug. This modification is crucial for extending the half-life of the aptamer in the bloodstream, reducing its clearance rate, and improving its bioavailability. PEGylation also reduces the immunogenicity of the aptamer, minimizing the risk of adverse immune responses and increasing the safety profile of the drug.
The PEG modifications in pegaptanib provide several key advantages. First, they significantly enhance the stability of the aptamer, allowing it to remain active in the bloodstream for a longer period. This increased stability is crucial for the drug's effectiveness in binding to and inhibiting VEGF165. Second, PEGylation improves the pharmacokinetic profile of the drug, increasing its half-life and bioavailability. This means that the drug can be administered less frequently, which is beneficial for patient convenience and compliance. Third, the PEG modifications reduce the immunogenicity of the aptamer, minimizing the risk of adverse immune responses and increasing the safety profile of the drug.
The role of the PEG modifications in pegaptanib is multifaceted. They protect the aptamer from degradation by nucleases, ensuring that it can reach its target in the eye. They also enhance the binding affinity of the aptamer to VEGF165, ensuring efficient and specific inhibition of VEGF activity. Additionally, the PEG modifications improve the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are essential for its therapeutic effectiveness. These modifications also reduce the immunogenicity of the aptamer, minimizing the risk of adverse immune responses.
Summary and Prospect
In summary, pegaptanib represents a significant advancement in the treatment of neovascular (wet) age-related macular degeneration (AMD). Its mechanism of action, involving the inhibition of VEGF165, has shown promising results in reducing the risk of vision loss and improving visual acuity in patients with wet AMD. Despite facing competition from newer and more effective therapies, pegaptanib has contributed to the understanding of the role of VEGF in the disease and paved the way for the development of advanced treatments. Future research may focus on improving delivery methods and exploring combination therapies to enhance its clinical utility. The sequence characteristics and chemical modifications of pegaptanib, including its PEGylation, contribute to its stability, specificity, and efficacy, making it a valuable tool in the management of wet AMD. However, the need for regular intravitreal injections and the management of side effects will continue to be important considerations in its clinical use. The ongoing and future clinical trials will provide further insights into the long-term safety and efficacy of pegaptanib, potentially offering new perspectives on its role in the treatment of wet AMD.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
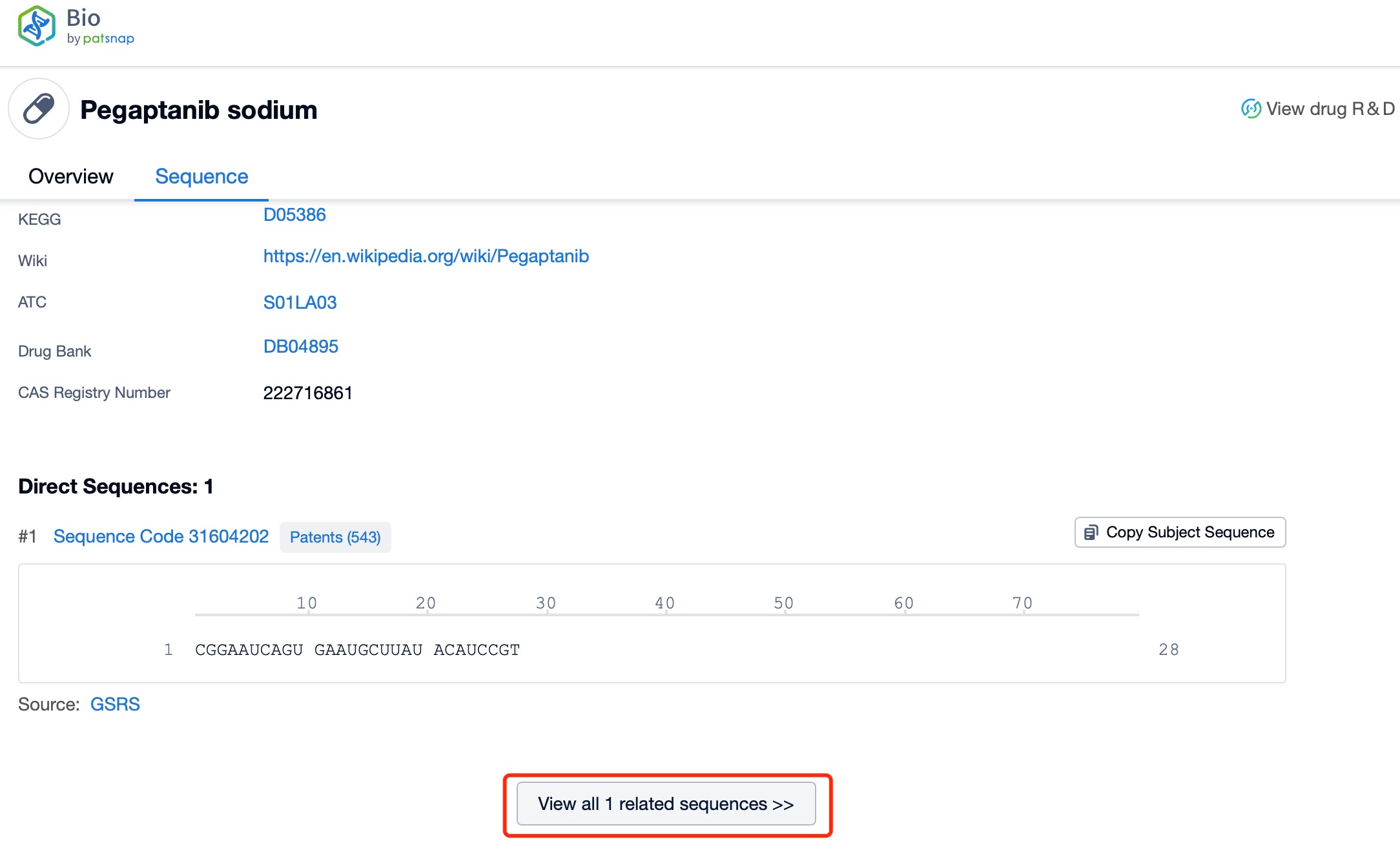
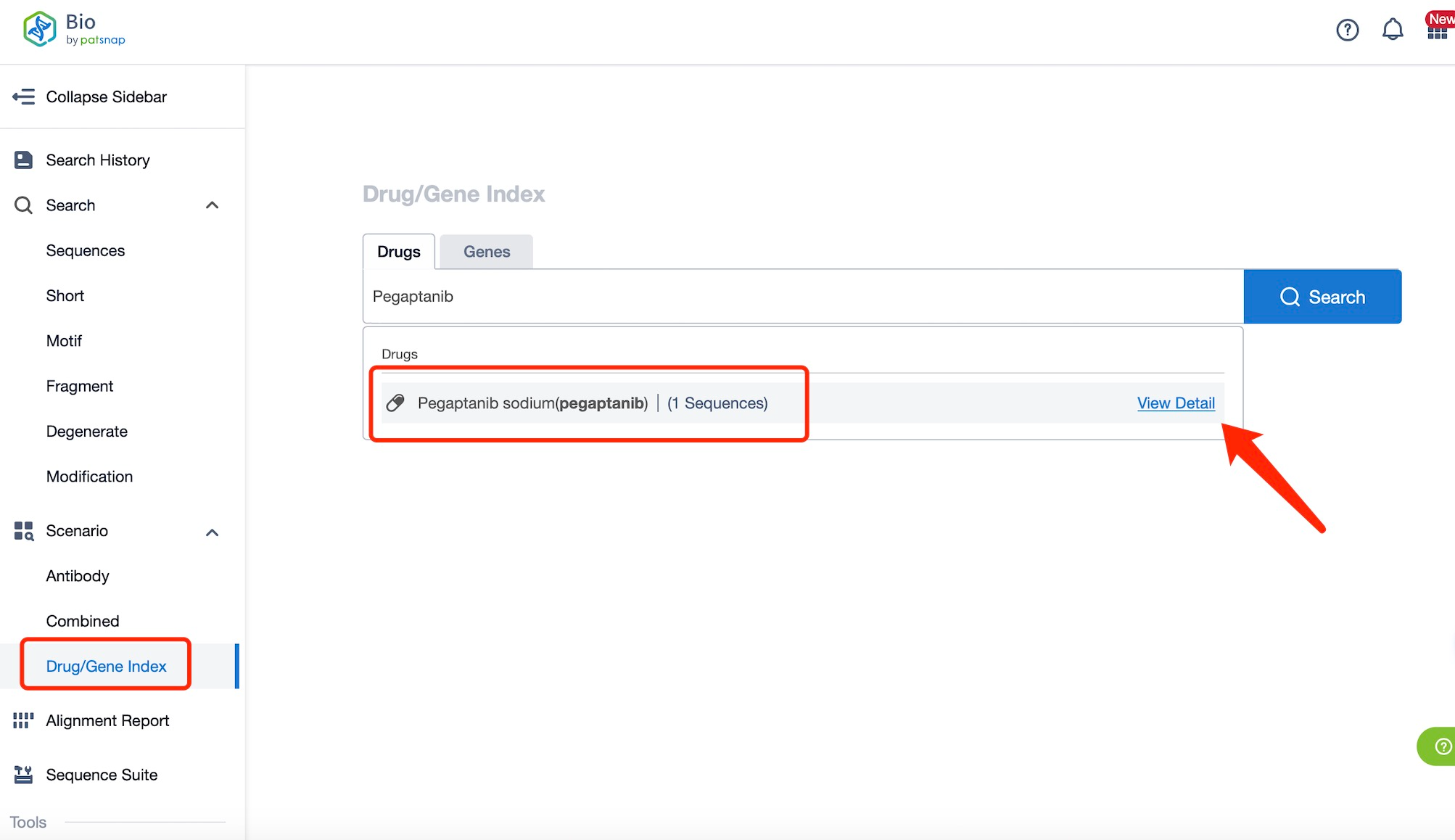
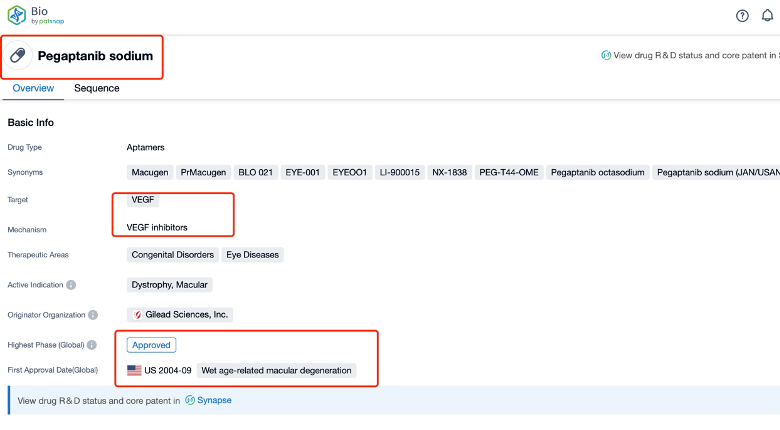
Taking pegaptanib as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' pegaptanib ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of pegaptanib.
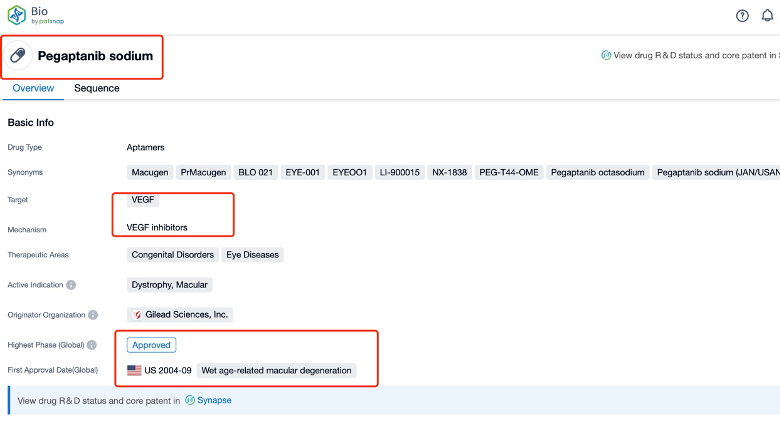
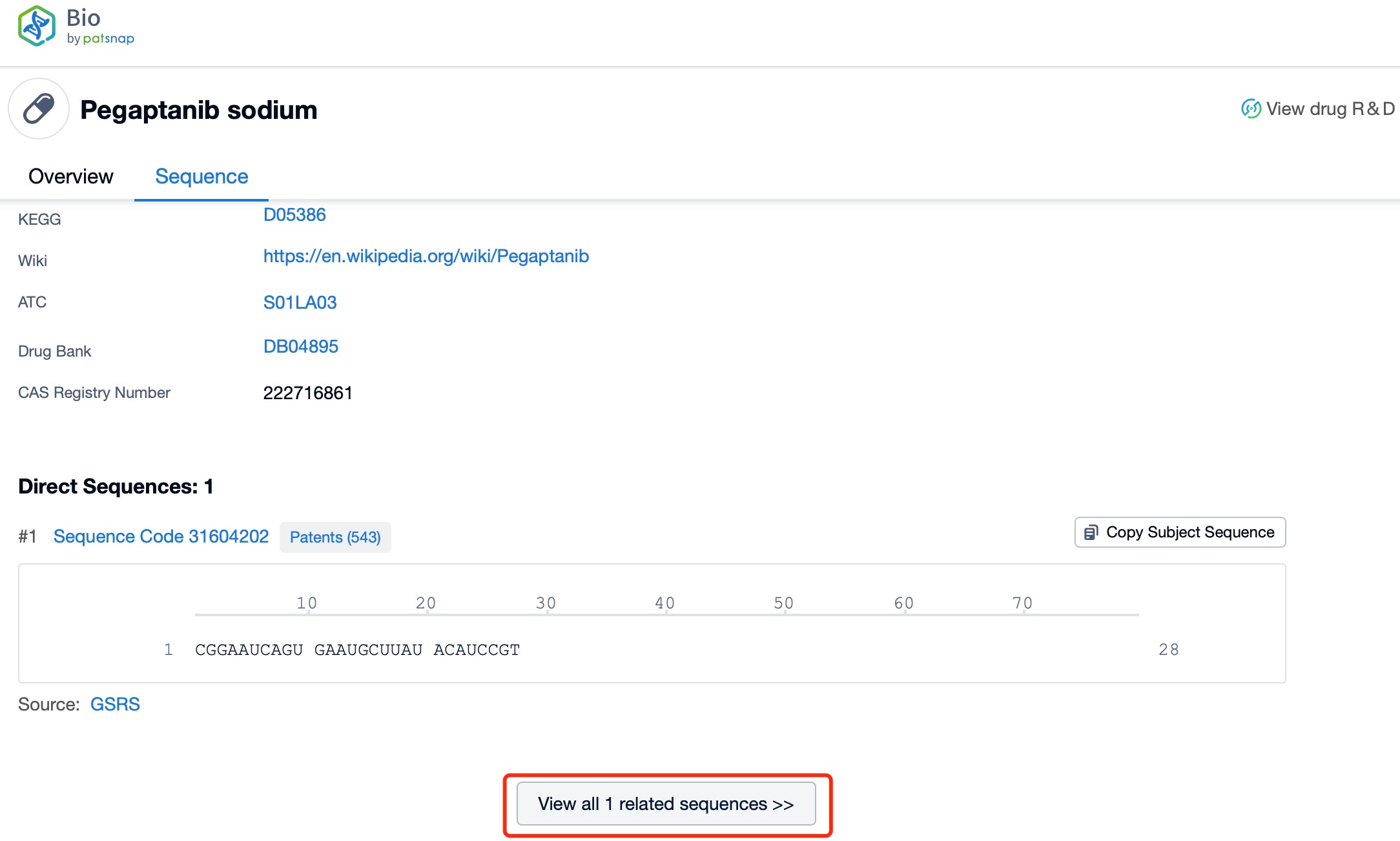
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
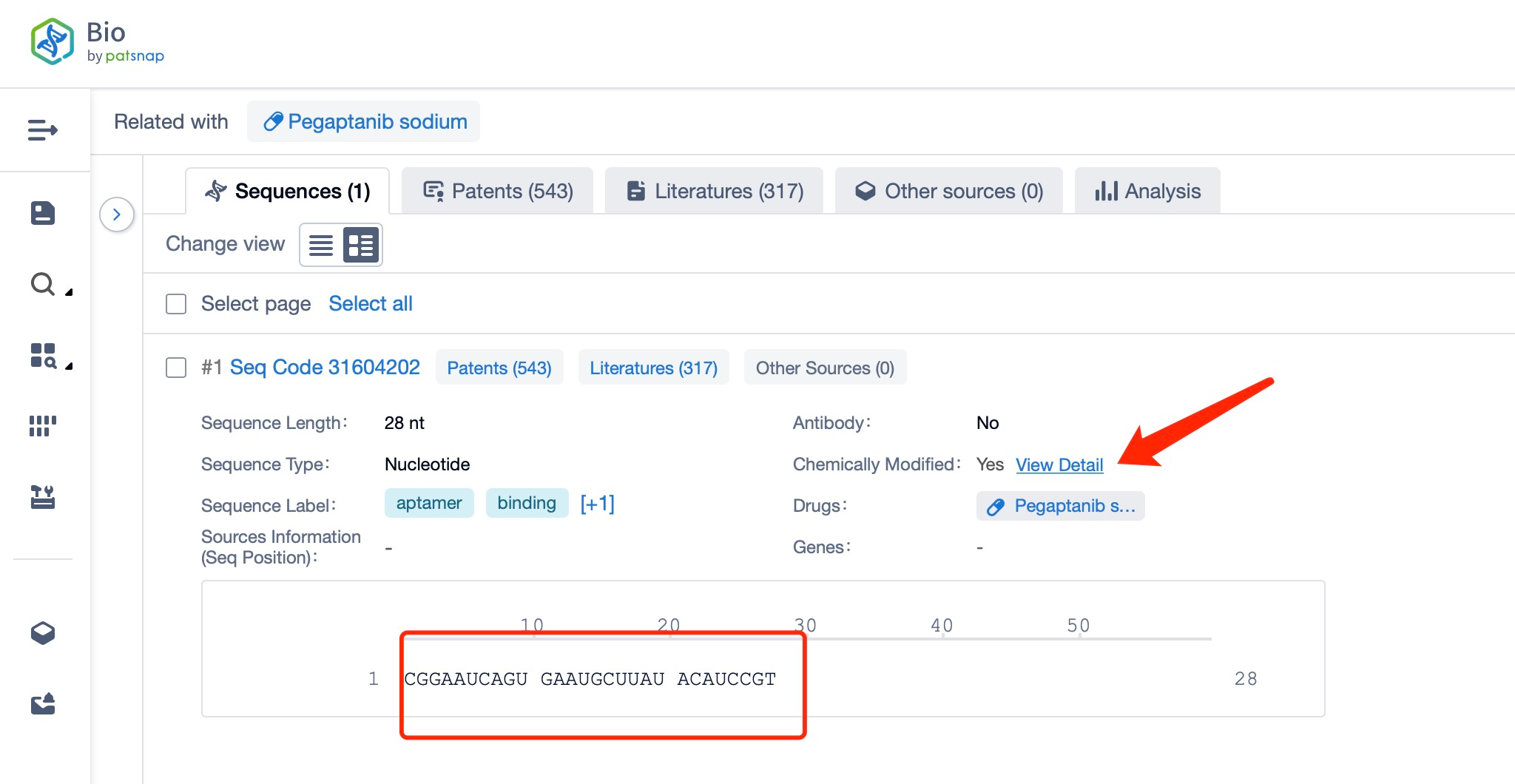
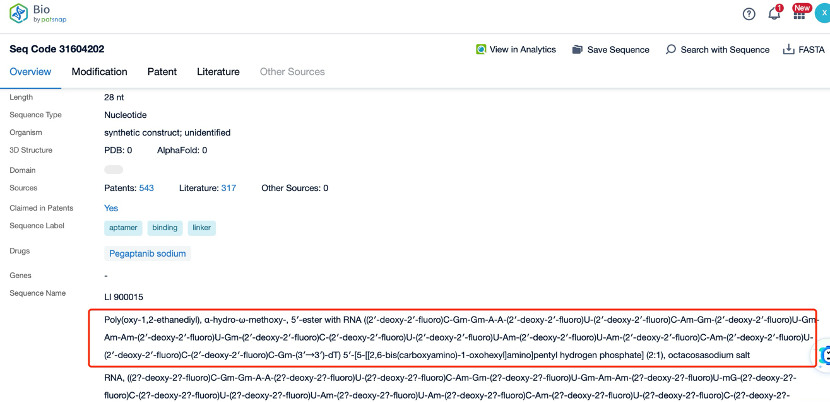
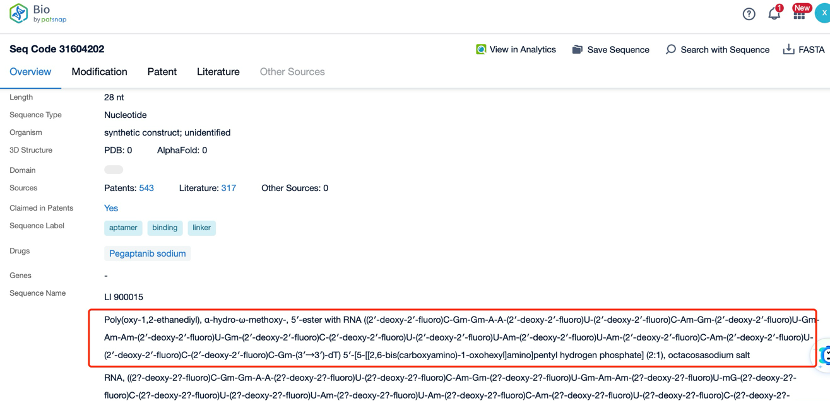
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.