Hydroxyprogesterone Caproate Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Hydroxyprogesterone Caproate's R&D Progress
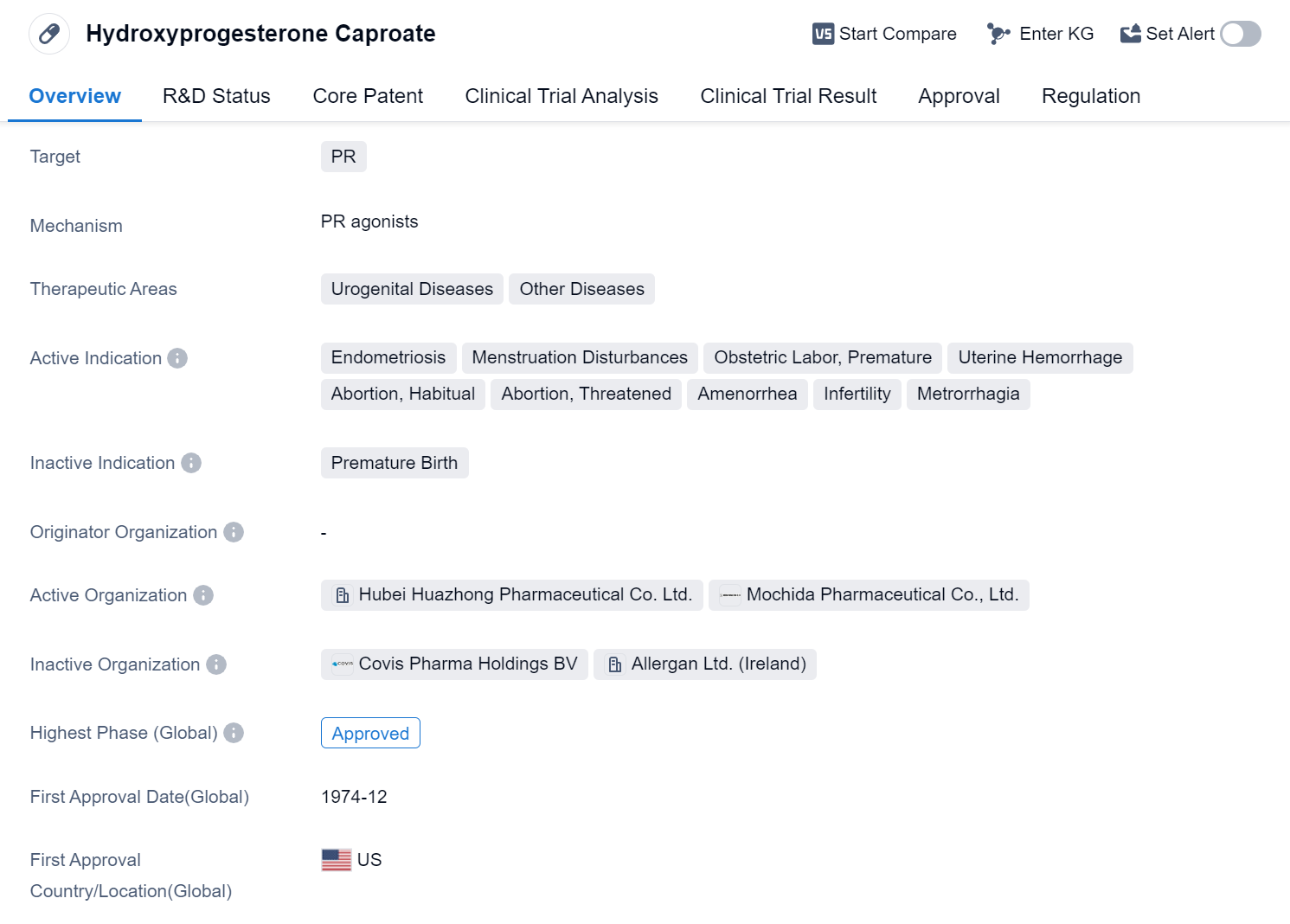
Hydroxyprogesterone Caproate is a small molecule drug that targets the progesterone receptor (PR). It is primarily used in the treatment of urogenital diseases and other diseases. The drug has been approved for various indications, including endometriosis, menstruation disturbances, obstetric labor, premature birth, uterine hemorrhage, habitual abortion, threatened abortion, amenorrhea, infertility, and metrorrhagia.
The highest phase of development for Hydroxyprogesterone Caproate is approved globally. It received its first approval in December 1974 in the United States. The drug has been granted accelerated approval and orphan drug status, indicating its potential to address unmet medical needs and rare diseases.
Hydroxyprogesterone Caproate is commonly used in the management of endometriosis, a condition where the tissue lining the uterus grows outside of it, causing pain and fertility issues. It is also prescribed for menstruation disturbances, such as irregular or heavy periods. Additionally, the drug is utilized to prevent premature birth by reducing the risk of preterm labor.
Uterine hemorrhage, both during pregnancy and outside of it, can be effectively treated with Hydroxyprogesterone Caproate. The drug is also used in cases of habitual abortion, where a woman experiences recurrent pregnancy losses, and threatened abortion, where there is a risk of miscarriage. Amenorrhea, the absence of menstrual periods, can be managed with this medication as well.
Infertility, a condition characterized by the inability to conceive, can be addressed with Hydroxyprogesterone Caproate. It helps regulate the menstrual cycle and promote ovulation, increasing the chances of successful conception. Metrorrhagia, abnormal uterine bleeding between periods, can also be controlled with this drug.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Hydroxyprogesterone Caproate: PR agonists
PR agonists are a type of medication that activate the progesterone receptor (PR) in the body. Progesterone is a hormone that plays a crucial role in the female reproductive system, particularly in regulating the menstrual cycle and supporting pregnancy. PR agonists mimic the effects of progesterone by binding to and activating the PR, leading to various physiological responses.
From a biomedical perspective, PR agonists are commonly used in the field of reproductive medicine. They can be prescribed to women who have irregular menstrual cycles, hormonal imbalances, or fertility issues. By activating the PR, these agonists help regulate the menstrual cycle, promote ovulation, and prepare the uterus for implantation of a fertilized egg. In some cases, PR agonists may also be used to support pregnancy by maintaining the uterine lining and preventing miscarriage.
It's important to note that PR agonists should only be used under medical supervision, as they can have potential side effects and interactions with other medications. The specific type and dosage of PR agonist prescribed may vary depending on the individual's condition and treatment goals.
Drug Target R&D Trends for Hydroxyprogesterone Caproate
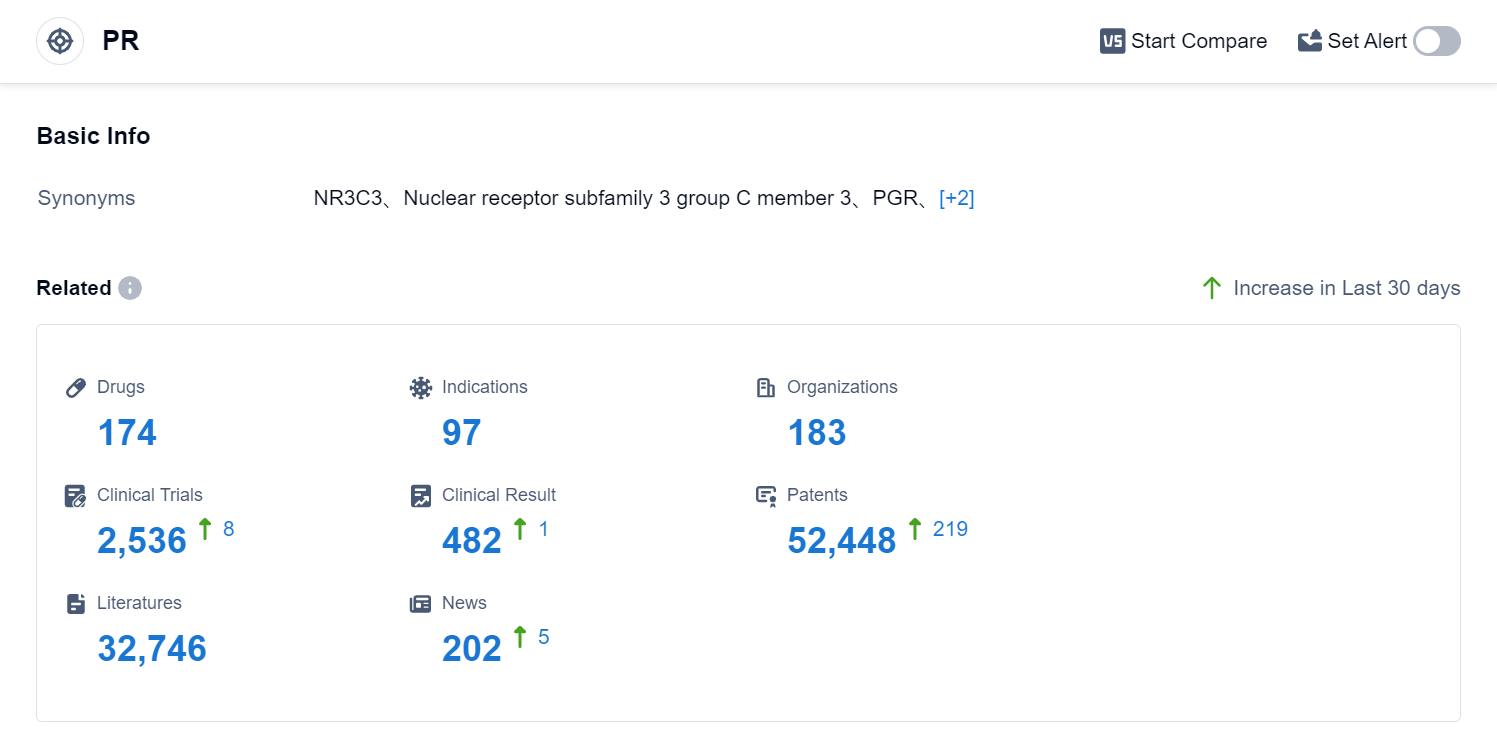
According to Patsnap Synapse, as of 1 Sep 2023, there are a total of 174 PR drugs worldwide, from 183 organizations, covering 97 indications, and conducting 2536 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of the target PR shows that Bayer AG is the leading company with the highest stage of development and the most approved drugs. The indications with the highest number of approved drugs are contraception, pregnancy, and osteoporosis (postmenopausal). Small molecule drugs are progressing rapidly, indicating intense competition in the pharmaceutical industry. The United States, China, the European Union, and Japan are the countries/locations with the fastest development under the target PR. China has shown significant progress in drug development. Overall, the target PR has a competitive landscape with multiple companies, diverse indications, and a focus on small molecule drugs. Future development in this area will likely involve further advancements in R&D and increased competition among pharmaceutical companies.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Hydroxyprogesterone Caproate is a well-established drug with a long history of use in the treatment of various urogenital diseases and other conditions. The drug's ability to target the progesterone receptor makes it a valuable therapeutic option for patients suffering from the mentioned indications.