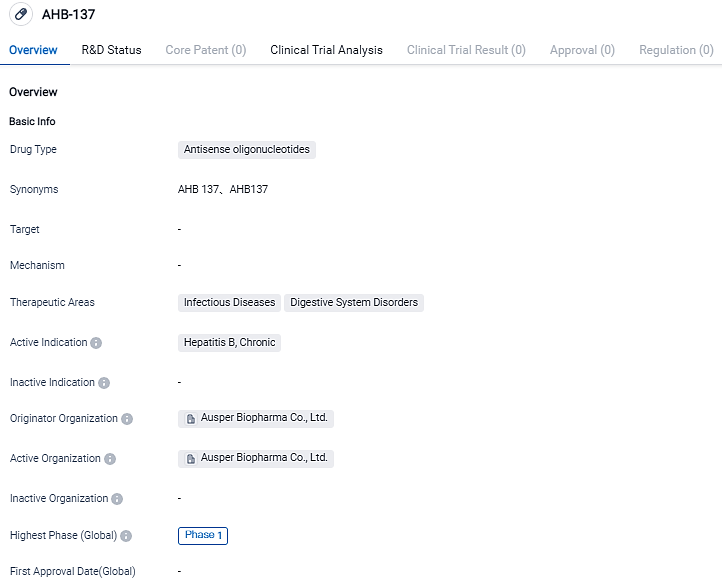
AusperBio Declares FDA Approval of IND Application for AHB-137 for Treating Chronic Hepatitis B
AusperBio Therapeutics, Inc. along with Ausper Biopharma Co., Ltd., a biotech corporation in the clinical stage devoted to the evolution of antiviral treatments and inoculations, with primary emphasis on delivering a functional remedy for persisting hepatitis B infection, have declared today that the FDA has sanctioned the IND application for testing of AHB-137 in clinics.
This trial in the America is a part of a widespread, randomized, double-blind, placebo-controlled research agenda, aiming to evaluate the security, endurance, pharmacokinetics, and preliminary effectiveness of AHB-137 in patients suffering from CHB.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The acceptance of our IND application by the FDA to kick off AHB-137's clinical appraisal for CHB patients in America is a significant step towards providing a possible functional solution for those battling HBV." stated AusperBio's CEO and Co-founder, Dr. Guofeng Cheng, "This important milestone marks a decisive moment for AusperBio, highlighting our continuous and extraordinary performance in propelling ground-breaking treatments towards CHB's functional cure."
Dr. Cheng Yong Yang, CSO and Co-founder of AusperBio emphasized, "We are dedicated to accelerating patient’s access to novel therapies. Our priority at this moment is closely coordinating with influential thought leaders to commence CHB patient-based research in the US."
About AusperBio.
AusperBio is a biopharmaceutical company in the clinical trial stage, with branches in the United States and China. The company is committed to creating pioneering remedies for the treatment of chronic hepatitis B. The company has invented a unique Med-Oligo™ ASO technology platform, significantly boosting the efficacy of designated therapies, not limited to liver-associated illnesses, but also potentially applicable to a spectrum of other diseases.
AusperBio’s approach involves merging its state-of-the-art oligonucleotide remedies with other pharmaceuticals including therapeutic antibodies and mRNA vaccines for addressing an array of unfulfilled medical requirements.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
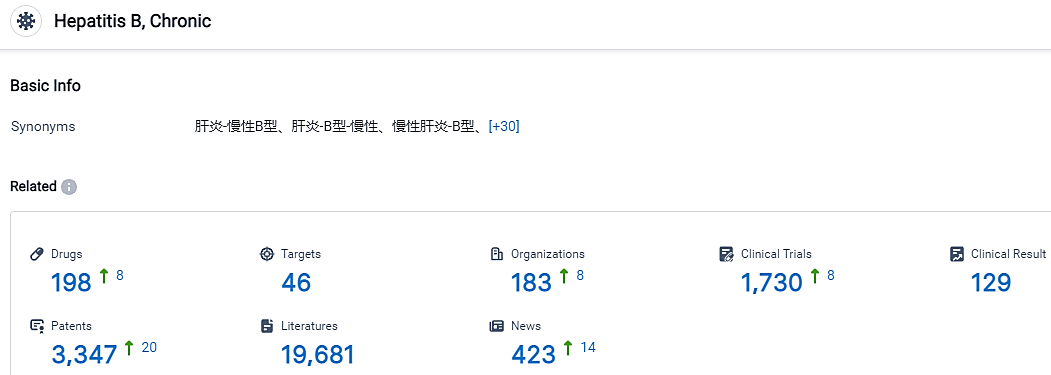
According to the data provided by the Synapse Database, As of September 4, 2023, there are 198 investigational drugs for the Chronic Hepatitis B, including 46 targets, 183 R&D institutions involved, with related clinical trials reaching 1730, and as many as 3347 patents.
Chronic Hepatitis B is a liver ailment that is believed to impact approximately 290 million individuals globally, leading to other long-term health issues like cirrhosis and hepatocellular carcinoma. Even though existing treatments can control HBV replication, attaining complete recovery is uncommon. Therefore, finding a treatment for CHB continues to be a pressing requirement.