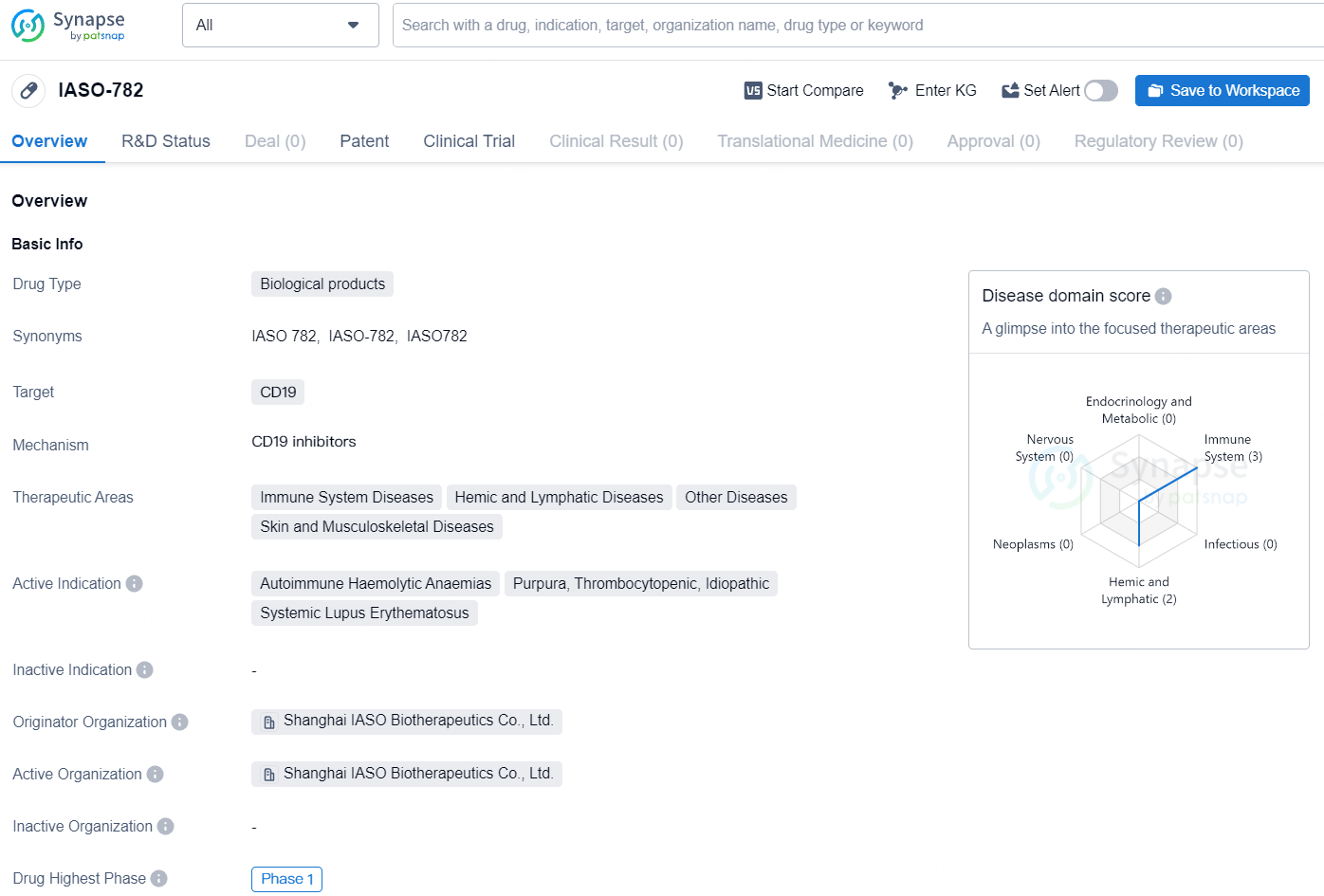
IASO Bio Gets NMPA Nod for IASO-782 IND in SLE Treatment
IASO Biotechnology, a biopharmaceutical firm committed to the discovery, development, manufacturing, and commercialization of innovative cell therapy and antibody solutions, announces the approval of the investigational new drug application for IASO-782 Injection by the National Medical Products Administration. IASO-782, a fully human monoclonal antibody that targets human CD19, is now approved for the treatment of a new indication — systemic lupus erythematosus.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Before receiving its latest approval, the IASO-782 Injection had already obtained IND approval in both China and the United States for treating immune thrombocytopenic purpura and warm autoimmune hemolytic anemia. The recent approval for Systemic Lupus Erythematosus (SLE) broadens the therapeutic applications of the IASO-782 Injection, showcasing IASO Bio’s innovative prowess in biopharmaceuticals and its deep understanding of patient requirements.
Dr. Jie Chen, Chief Medical Officer of IASO Bio, remarked: “The IND approval from the US Food and Drug Administration covers four autoimmune indications, including SLE. IASO Bio remains committed to working alongside our peers to further advance innovation and development in autoimmune disease treatment, thereby providing substantial clinical benefits to patients through cutting-edge therapies.”
IASO-782 Injection is a fully human monoclonal antibody targeting human CD19, engineered with Fc mutations to enhance ADCC function while preserving other Fc functions like ADCP. The B cell surface antigen CD19 is expressed during B cell development, from pre-B cells to plasmablasts, and is also found in some plasma cells. Numerous autoimmune diseases, including ITP and AIHA, are predominantly driven by auto-reactive antibodies produced by pathogenic B cells and plasma cells.
IASO-782 effectively depletes CD19+ B cells, plasmablasts, and certain plasma cells, thereby reducing or eliminating auto-reactive antibodies generated by these cells. This gives IASO-782 the potential to treat various autoimmune diseases linked with auto-reactive antibodies. Being a fully human antibody, IASO-782 has low immunogenicity and is less likely to induce anti-drug antibodies upon repeated use. This characteristic is advantageous for treating autoimmune diseases that necessitate long-term, repeated administration.
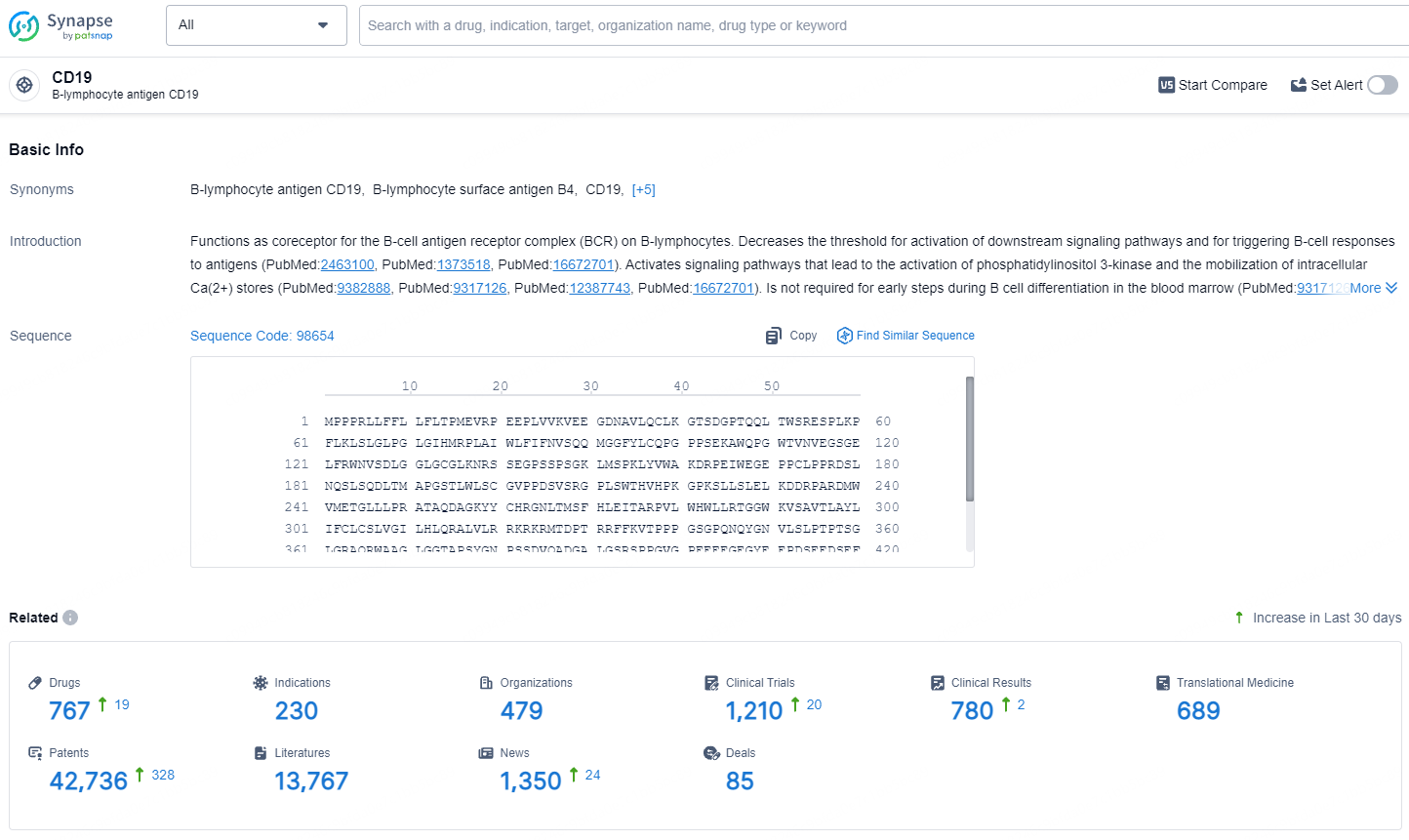
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 31, 2024, there are 767 investigational drugs for the CD19 targets, including 230 indications, 479 R&D institutions involved, with related clinical trials reaching 1210, and as many as 42736 patents.
IASO-782 represents a significant advancement in addressing unmet medical needs in the aforementioned therapeutic areas. As the drug progresses through clinical trials, its potential in treating diseases such as systemic lupus erythematosus and autoimmune haemolytic anaemias is an area of interest for further observation and research. The attainment of Phase 1 status in clinical trials underscores the commitment to advancing IASO-782 towards potential regulatory approval and subsequent availability for patients in need.