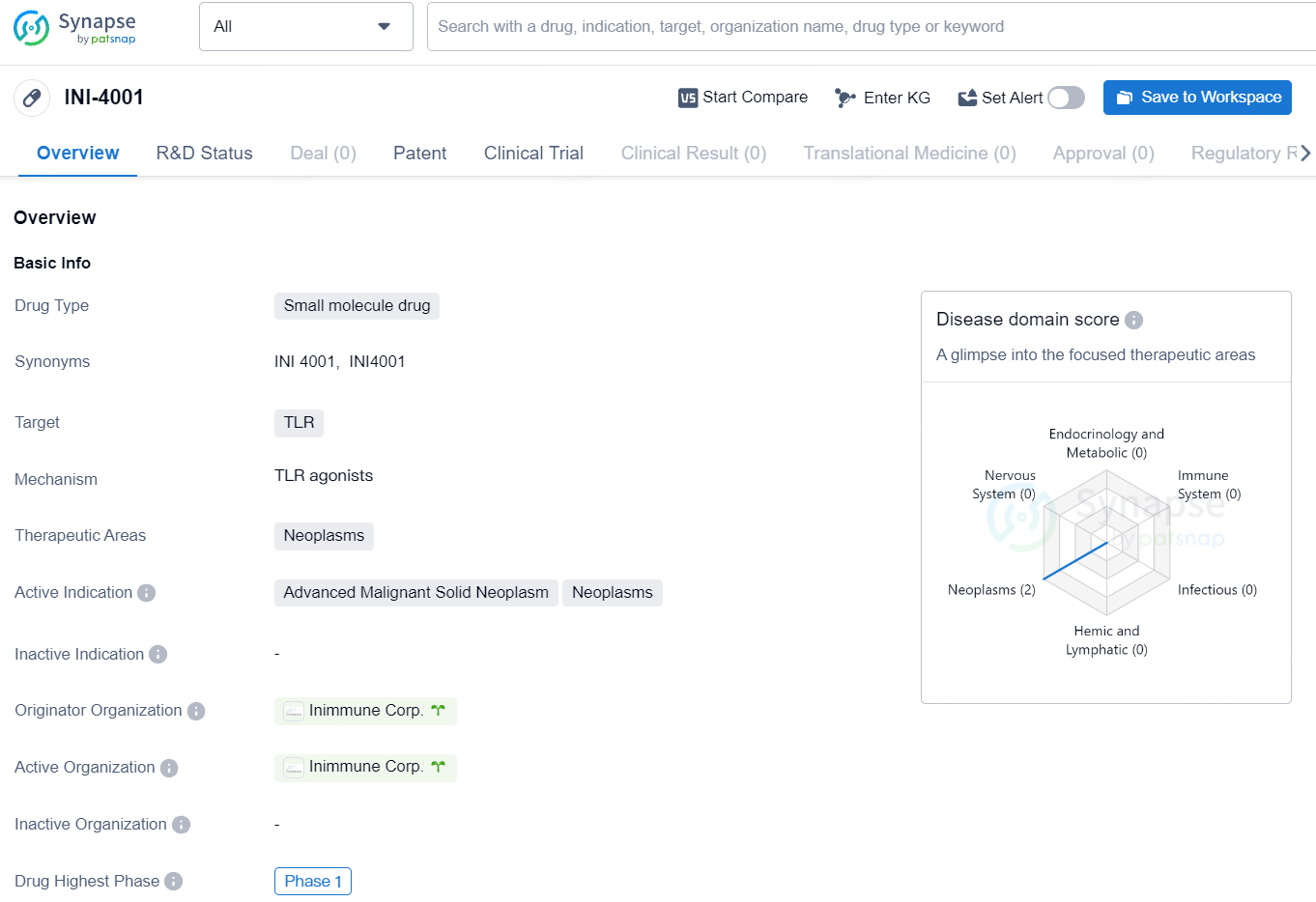
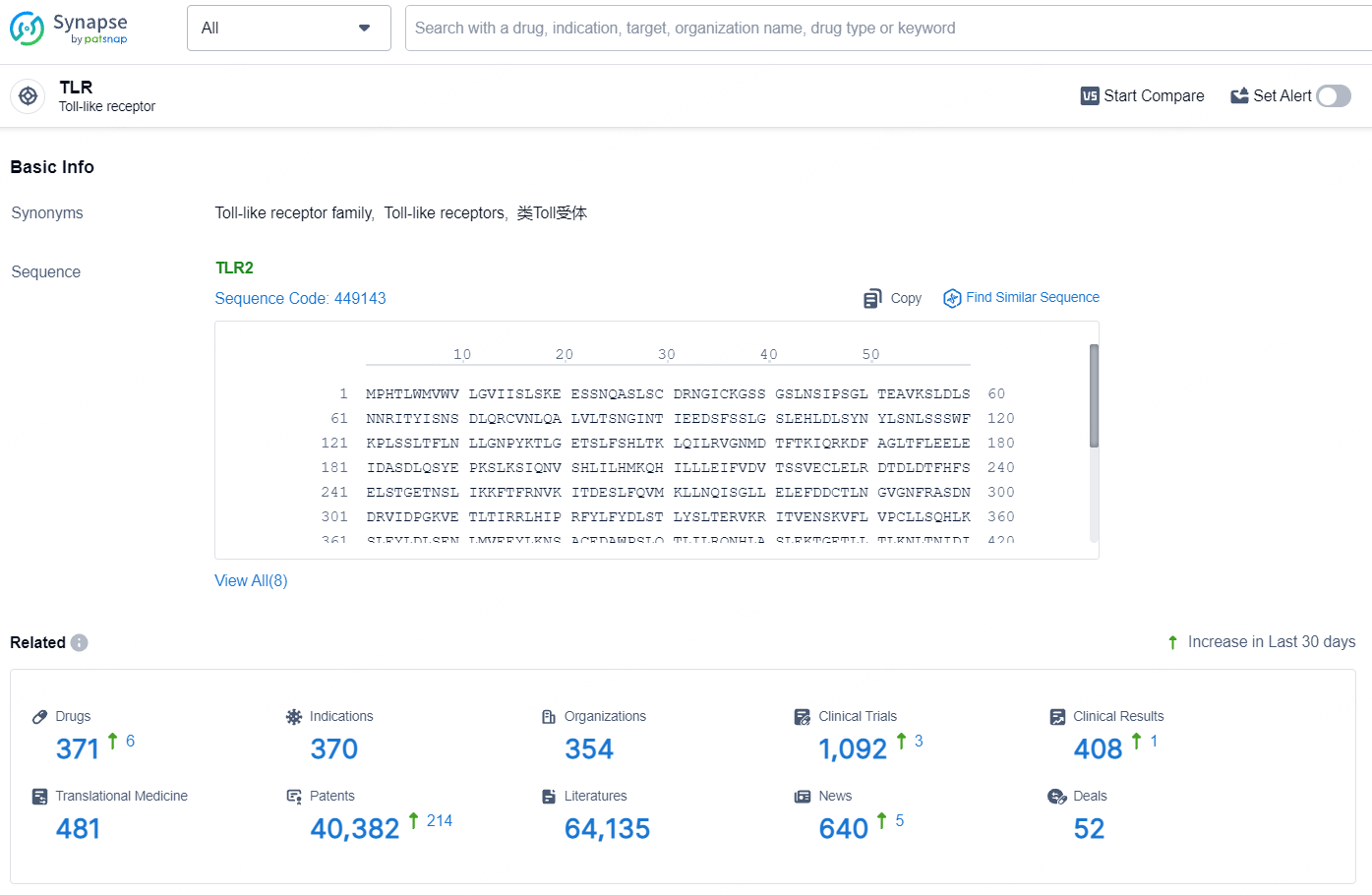
Initial Cancer Patient Treated with Inimmune's TLR7/8 Agonist INI-4001 in Phase 1 Trial
Inimmune, a biotechnology firm at the clinical stage specializing in the creation of novel immunotherapies, vaccines, and vaccine adjuvants, has declared that the initial patient has been administered in its Phase 1 single ascending dose trial of INI-4001, targeting individuals with advanced solid tumors.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This is an open-label, multiple-ascending dose study with two parts: dose ranging and cohort expansion of INI-4001 in individuals with advanced solid tumors. The trial marks the first human application of INI-4001, Inimmune’s proprietary TLR7/8 agonist incorporated within a nanoparticle delivery system, and is structured into two phases.
During Phase 1a, single-dose escalation cohorts will receive monotherapy INI-4001 to assess pharmacokinetics (PK), safety, and tolerability. This is followed by Phase 1b, where patients who either progressed or have stable disease after three cycles of INI-4001 will be eligible to receive a combination treatment of INI-4001 alongside an anti-PD-1 or anti-PD-L1 immunotherapy.
INI-4001 has proven to be effective as a standalone treatment and when combined with anti-PD-1 checkpoint therapy in pre-clinical studies involving syngeneic mouse tumor models (LLC, MC38, and B16F10). Additionally, INI-4001 has demonstrated the ability to induce cytokine IFNα production and activate antigen-presenting cells, which in turn promotes downstream T cell activation in vivo. Given that only a small fraction of patients currently benefit from checkpoint inhibitor monotherapy, INI-4001 could potentially serve as an ideal combination agent with these inhibitors.
Alan Joslyn, CEO of Inimmune, commented, “The commencement of INI-4001 clinical trials is a significant milestone for both our company and oncology patients. INI-4001, either as a monotherapy or in combination with checkpoint therapy, holds promise as a novel therapeutic option for physicians.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 31, 2024, there are 371 investigational drugs for the TLR7/8 target, including 370 indications, 354 R&D institutions involved, with related clinical trials reaching 1092, and as many as 40382 patents.
INI-4001 represents a novel approach to addressing neoplasms by targeting TLRs with a small molecule drug. Its early-stage status in Phase 1 clinical development indicates the need for further research and evaluation to determine its safety and potential benefits for patients with advanced malignant solid neoplasms.