Icotrokinra Shows Promising Skin Improvement and Safety in Phase 3 Trials
Johnson & Johnson (NYSE: JNJ) has revealed encouraging topline findings from the ICONIC-LEADa study, a crucial Phase 3 trial evaluating icotrokinra (JNJ-2113). This compound is the first targeted oral peptide designed to selectively inhibit the IL-23 receptor in individuals aged 12 and older who suffer from moderate to severe plaque psoriasis (PsO). The Phase 3 trial successfully achieved its co-primary endpoints, including the Psoriasis Area and Severity Index (PASI) 90b and Investigator's Global Assessment (IGA) scores of 0/1c at the 16-week mark, with response rates continuing to show improvement up to week 24.
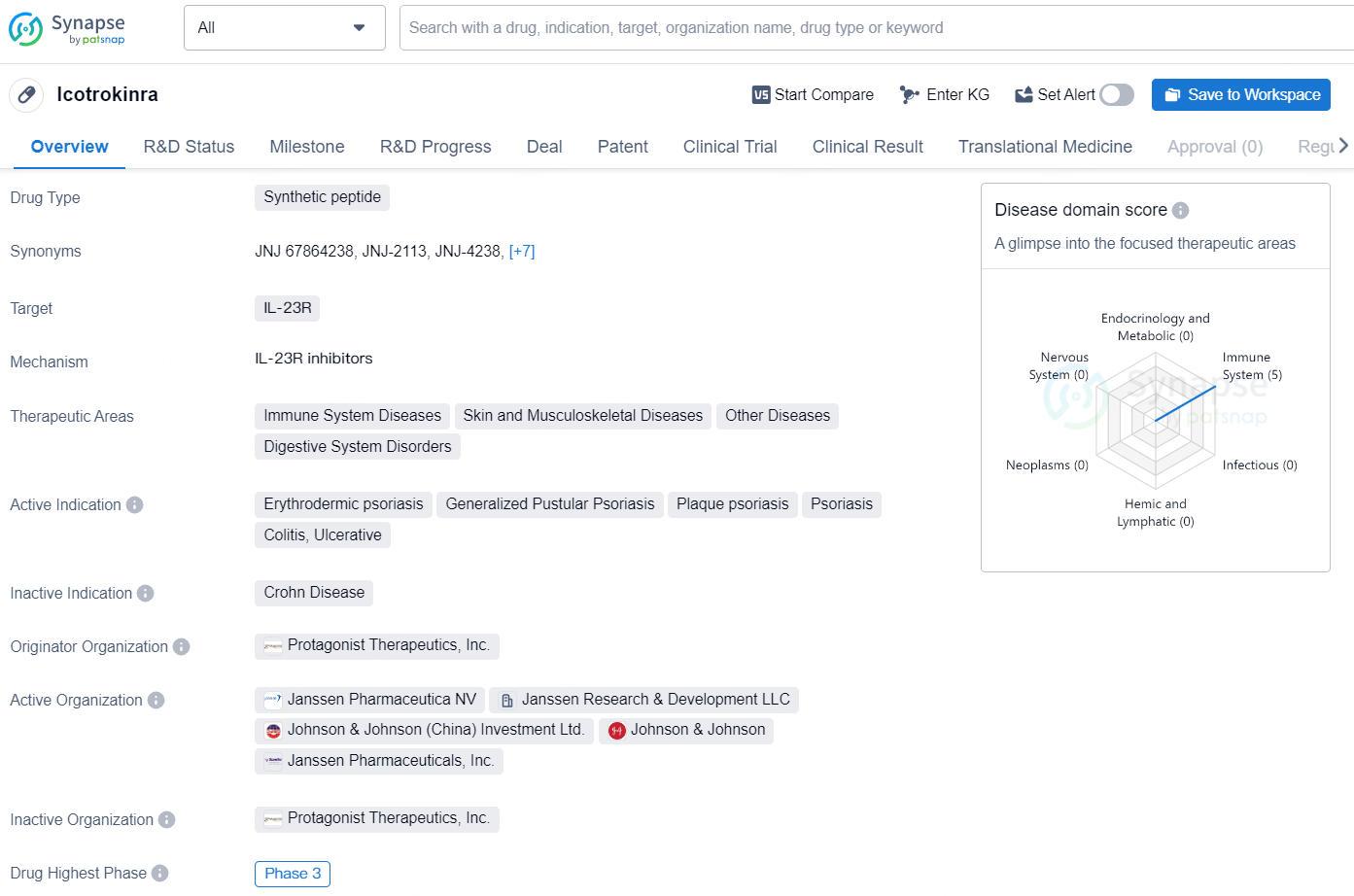
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Once-daily icotrokinra demonstrated notable skin improvement compared to placebo in adults and teenagers suffering from moderate to severe plaque psoriasis. By week 16, almost two-thirds (64.7%) of individuals receiving icotrokinra achieved IGA scores of 0/1 (indicating clear or almost clear skin), while 49.6% attained PASI 90; in contrast, these figures for the placebo group were 8.3% and 4.4%, respectively.1 Response rates continued to rise by week 24, with 74.1% of icotrokinra-treated patients reaching IGA scores of 0/1 and 64.9% achieving PASI 90.1 Safety results were in line with those observed in the Phase 2 FRONTIER 1 and 2 trials, showing comparable occurrences of adverse events (AEs) between the icotrokinra and placebo groups, with 49.3% and 49.1% of patients, respectively, reporting treatment emergent adverse events (TEAEs) at week 16.
Additionally, favorable topline outcomes from the Phase 3 ICONIC-TOTALd study indicated that the once-daily dosage of icotrokinra successfully met the primary goal of achieving IGA scores of 0/1 at week 16 when compared to placebo. Detailed findings from ICONIC-LEAD and ICONIC-TOTAL are being compiled for forthcoming medical conferences and will be submitted to health authorities shortly.
“We are thrilled to observe remarkable Phase 3 findings with once-daily icotrokinra treatment, consistent with our Phase 2 trial of this pioneering targeted oral peptide that specifically inhibits the IL-23 receptor,” stated Liza O’Dowd, Vice President and Lead of the Immunodermatology Disease Area at Johnson & Johnson Innovative Medicine. “A significant number of patients dealing with moderate to severe plaque psoriasis qualify for, yet do not receive, advanced treatment options. Icotrokinra holds the promise of providing a once-daily oral therapy that may meet the needs and preferences of individuals affected by plaque psoriasis.”
Further investigations within the Phase 3 ICONIC clinical development program are ongoing, encompassing ICONIC-ADVANCE 1 and ICONIC-ADVANCE 2, which will assess the safety and effectiveness of icotrokinra against both placebo and deucravacitinib in moderate to severe plaque psoriasis. The Phase 3 ICONIC-PsA initiative, focusing on psoriatic arthritis, is set to commence in early 2025.
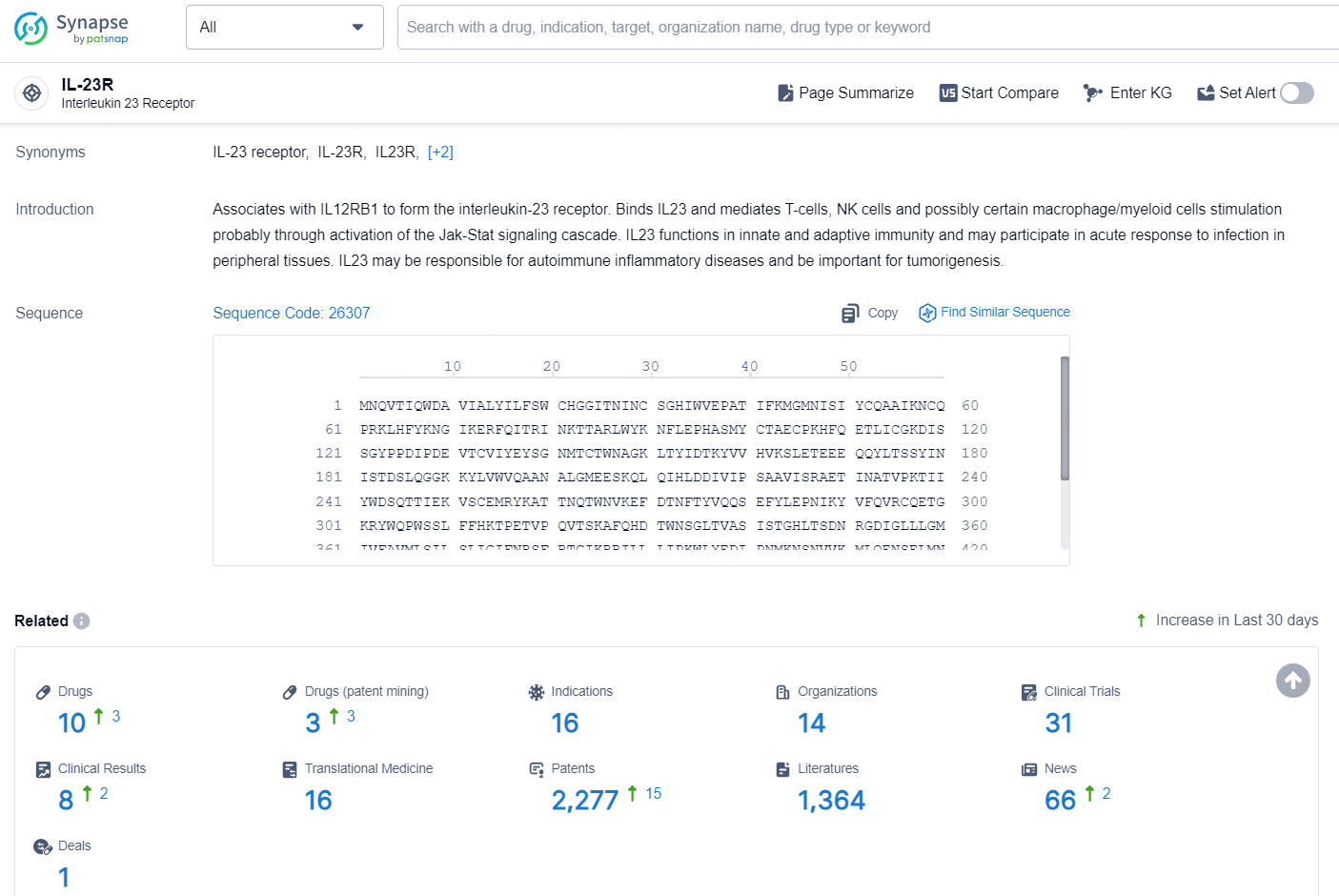
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 25, 2024, there are 10 investigational drugs for the IL-23R target, including 16 indications, 14 R&D institutions involved, with related clinical trials reaching 31, and as many as 2277 patents.
Icotrokinra is a synthetic peptide drug that targets IL-23R and is being developed for the treatment of a variety of therapeutic areas, including immune system diseases, skin and musculoskeletal diseases, other diseases, and digestive system disorders.