Virion Therapeutics Reveals Initial Clinical Responses of HBV Checkpoint Modifier VRON-0200 at AASLD Meeting
Virion Therapeutics, LLC, a biotechnology firm in the clinical development stage, specializing in innovative T cell-based immunotherapies, announced encouraging data related to immunologic responses, as well as ongoing safety and tolerability, and anti-HBV immune reactions from the initial human trial of its groundbreaking, first-in-class checkpoint modifier immunotherapy, VRON-0200, aimed at achieving a functional cure for HBV. This information was presented by Professor Grace Wong, M.D., from the Chinese University of Hong Kong, during a late-breaking poster session at The Liver Meeting® 2024 (organized by the American Association for the Study of the Liver) in San Diego, California, USA.
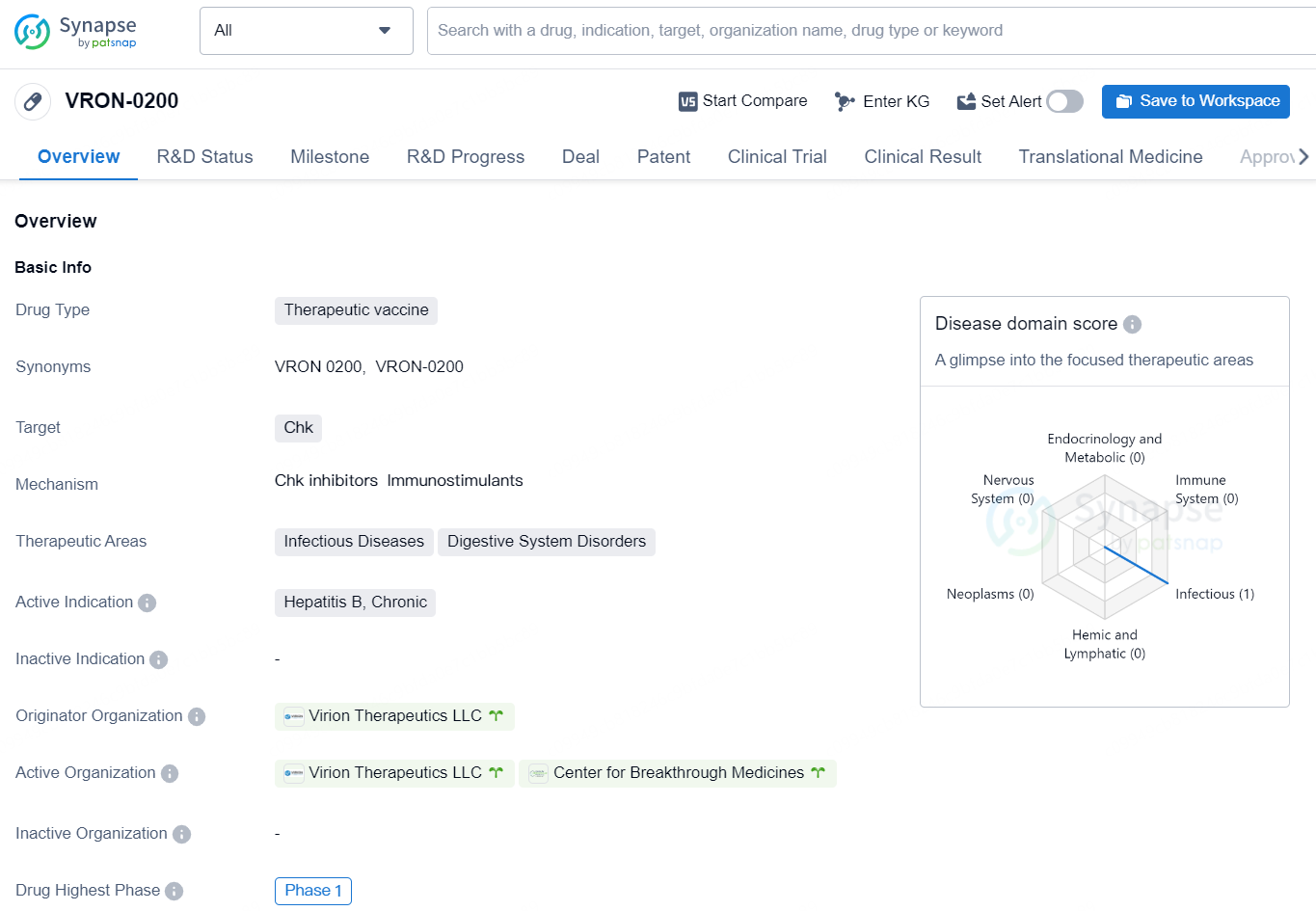
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 1b study involving the initial cohort of 25 patients suffering from chronic hepatitis B infection, all undergoing nucleos(t)ide antiviral treatment and receiving a solitary intramuscular injection of VRON-0200, confirmed its safety profile and tolerability. There were no major adverse effects noted, and laboratory assessments, which included tests for liver function, showed no significant abnormalities. Among the 22 patients monitored immunologically for a minimum of 28 days, a notable 5.5-fold elevation in T cell responses was recorded in 7 individuals (32%) following vaccination, with a majority sustaining these responses above baseline levels up to day 91. Additionally, four patients exhibited declines in HBV surface antigen (HBsAg) ranging from -0.4 to -2.3 log10 IU/mL after a single injection.
Professor Wong remarked, “Restoring immune function in patients chronically infected with HBV is crucial to cease treatment and eradicate the infection. These findings are extremely encouraging for this patient population, the majority of whom were infected at birth. The single injection of VRON-0200 not only stimulated T cell responses and reduced serum HBsAg but also demonstrated a favorable safety profile. The documented reductions in HBsAg are particularly intriguing, as VRON-0200 does not directly target HBsAg, implying that the patients may be gaining some immune control over the virus. I am eager to see further results and extended follow-up from this investigation.”
Dr. Sue Currie, COO of Virion and an author of the study, stated, “These findings underscore the potential of VRON-0200 as a straightforward and accessible immunotherapy that spares interferon, either alone or in combination, for achieving a functional cure for HBV. VRON-0200 is the first novel immunotherapy aside from monoclonal antibody checkpoint inhibitors and pegylated interferon to reduce HBsAg without directly addressing it. Following the positive data, we are now exploring our first combination therapy involving VRON-0200 alongside elebsiran (siRNA) and tobevibart (mAb), aiming to enhance responses and offer a cure for the nearly 300 million people living with chronic HBV. We anticipate presenting additional findings in early 2025.”
Professor Ed Gane, M.D., from the University of Auckland and a study author, added, “Non-immunologic methods for the removal of HBsAg, such as siRNA and small molecules, have not proven effective in achieving durable off-treatment responses or restoring the necessary immunity to manage HBV infection. The excitement surrounding these results stems from the fact that a single dose of VRON-0200 produced declines in HBsAg, suggesting evidence of immune control against HBV in these patients. These results, along with forthcoming data from the ongoing combination cohort trial, will guide future approaches for therapies aimed at achieving a functional cure.”
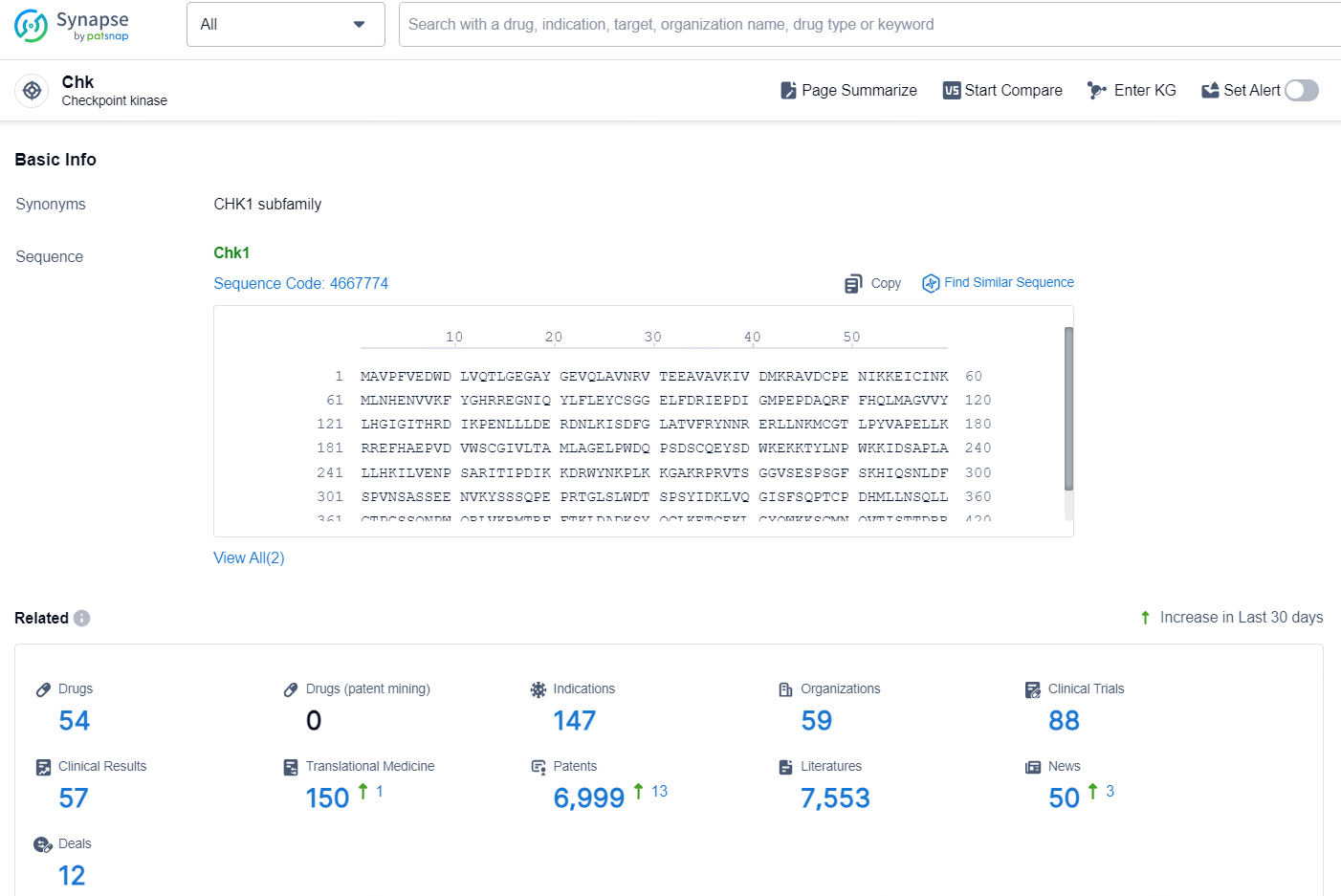
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 25, 2024, there are 54 investigational drugs for the Chk target, including 147 indications, 59 R&D institutions involved, with related clinical trials reaching 88, and as many as 6999 patents.
The drug VRON-0200 is a therapeutic vaccine being developed by Virion Therapeutics LLC. It is designed to target Chk and is intended for the treatment of Hepatitis B, Chronic, within the therapeutic areas of Infectious Diseases and Digestive System Disorders.