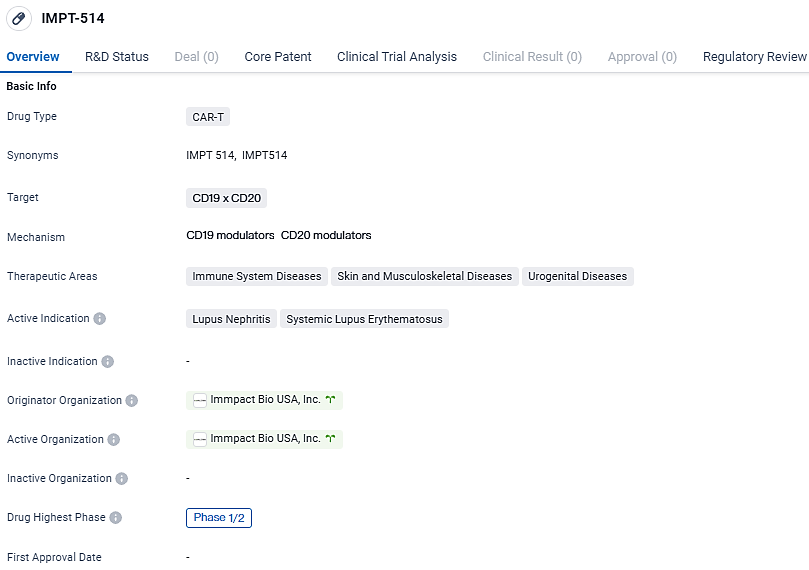
ImmPACT Bio Secures $8M CIRM Grant for Dual-Target CAR T Therapy Trial in Lupus
ImmPACT Bio USA, Inc. has disclosed the reception of a substantial research grant amounting to $8 million. This funding, bestowed by the California Institute for Regenerative Medicine, is allocated specifically to support ImmPACT Bio’s current research, particularly their Phase 1b/2 clinical trial. The trial is focused on the assessment of the innovative therapeutic agent IMPT-514, which is being investigated for its potential efficacy in addressing the challenging conditions of refractory lupus nephritis and systemic lupus erythematosus.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
IMPT-514 represents a groundbreaking therapy, potentially the first of its kind, as a dual-specificity CAR T-cell treatment targeting CD19/CD20 markers with the intent to deeply purge self-targeting immune cells. Awarded the status of Fast Track by the FDA, this treatment is poised to address both active and treatment-resistant forms of LN as well as SLE.
"CAR T-cell treatments have revolutionized the management of hematologic malignancies. Exploring their use in autoimmune disease management is an area filled with potential," commented Dr. Abla Creasey, the Therapeutic Development Vice President at CIRM.
"We're excited to extend financial support to ImmPACT Bio in their innovative efforts to take CAR T-cell therapy beyond cancer treatment. We are optimistic that their strategy with IMPT-514, intended as a solitary treatment, has considerable promise for redefining the clinical management and care for patients with severe, treatment-resistant lupus," further stated Dr. Creasey.
Kathleen A. Arntsen, President and CEO of Lupus and Allied Diseases Association, Inc., expressed, "Given lupus’s variability, each patient presents a unique case. We lack a universal treatment strategy for individuals as complex as lupus patients like myself. What offers relief to one might not to another, hence, doctors need a diverse range of treatment options at their disposal for addressing lupus and its associated renal complications."
Currently, IMPT-514 is undergoing a Phase 1b/2 clinical study featuring an ascending dose framework. This trial is enrolling participants struggling with stubborn, active SLE who have previously failed to respond to at least two conventional treatment regimens and possess a SLEDAI-2K score higher than 8. The initial Phase 1 section is focusing exclusively on individuals with active, biopsy-proven, proliferative LN. The subsequent Phase 2 segment will expand to include a broader cohort of patients, those with and without active proliferative LN.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
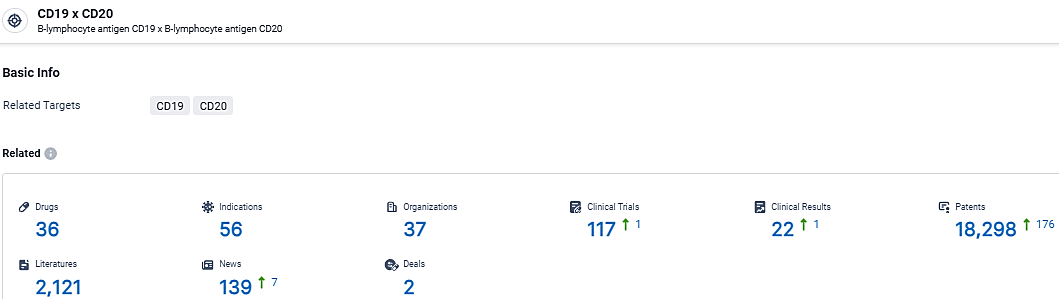
According to the data provided by the Synapse Database, As of March 4, 2024, there are 36 investigational drugs for the CD19/CD20 target, including 56 indications, 37 R&D institutions involved, with related clinical trials reaching 117, and as many as 18298 patents.
IMPT-514 is a CD19/CD20-targeting chimeric antigen receptor T-cell therapy that utilizes a potent bispecific CAR and a 4-1BB costimulatory domain. The drug is currently in Phase 1/2 of development and has been granted Fast Track designation.