Progress in the Discovery of New Drugs Targeting GPCR Class for Gastric Cancer
Gastric Cancer (GC), also known simply as GC, is one of the most lethal malignancies worldwide, with over one hundred million new cases reported in 2022, leading to the death of 783,000 individuals. Globally, the incidence and mortality rates of gastric cancer have been on the decline in most regions over the past few decades. This considerable change is largely attributed to economic development and improved practices in food preservation associated with refrigeration technology during food transport and storage.
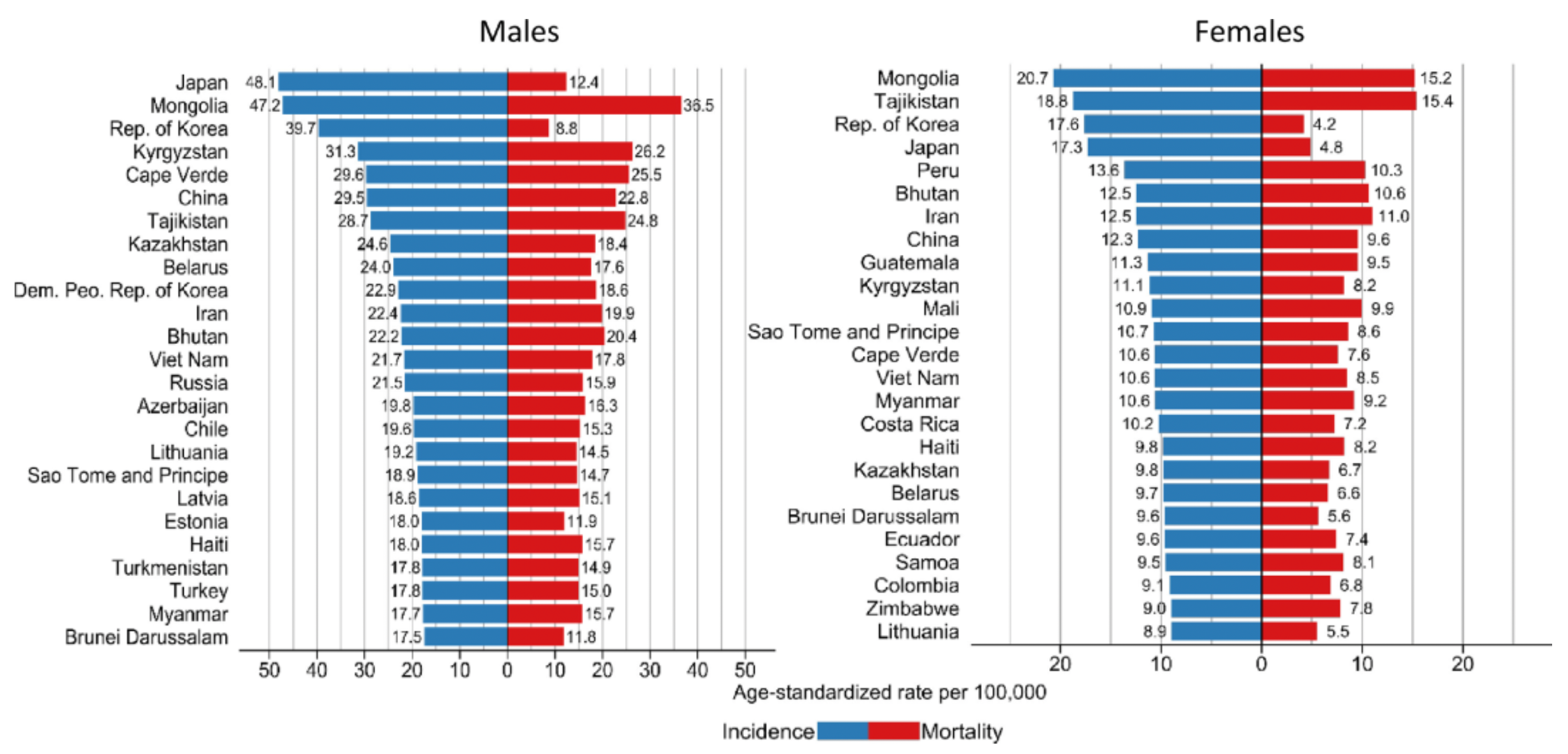
In 2020, it was estimated that there were 1.1 million cases of stomach cancer diagnosed globally, with 720,000 in males and 370,000 in females. Stomach cancer ranks among the top three most common cancers in 19 countries worldwide and is the most common cancer in four countries: Bhutan, Kyrgyzstan, Cape Verde, and Tajikistan. Sixty percent of all stomach cancer cases occur in East Asia, with China alone accounting for 43.9% of cases. The incidence of stomach cancer is higher in males, with 66% of all cases occurring in men. There are significant differences in the incidence rates across different regions; in several African countries, the incidence rate is less than 5 cases per 100,000 person-years in males, whereas in East Asian countries, it exceeds 30 cases per 100,000 person-years. This indicates that factors such as gender, region, and level of economic development have a significant impact on the incidence of stomach cancer. Moreover, in high-incidence areas such as East Asia, especially China, the prevention and control of stomach cancer represent a considerable challenge.
In 2020, it was estimated that there were 1.1 million cases of stomach cancer diagnosed globally, with 720,000 in males and 370,000 in females. Stomach cancer ranks among the top three most common cancers in 19 countries worldwide and is the most common cancer in four countries: Bhutan, Kyrgyzstan, Cape Verde, and Tajikistan. Sixty percent of all stomach cancer cases occur in East Asia, with China alone accounting for 43.9% of cases. The incidence of stomach cancer is higher in males, with 66% of all cases occurring in men. There are significant differences in the incidence rates across different regions; in several African countries, the incidence rate is less than 5 cases per 100,000 person-years in males, whereas in East Asian countries, it exceeds 30 cases per 100,000 person-years. This indicates that factors such as gender, region, and level of economic development have a significant impact on the incidence of stomach cancer. Moreover, in high-incidence areas such as East Asia, especially China, the prevention and control of stomach cancer represent a considerable challenge.
However, unlike the previously reported continuous decline in the overall incidence rate across all age groups, recent reports indicate an increase in the incidence of gastric cancer among young adults (under the age of 50), particularly a noticeable growth in the incidence of non-cardia tumors among the more affluent population.
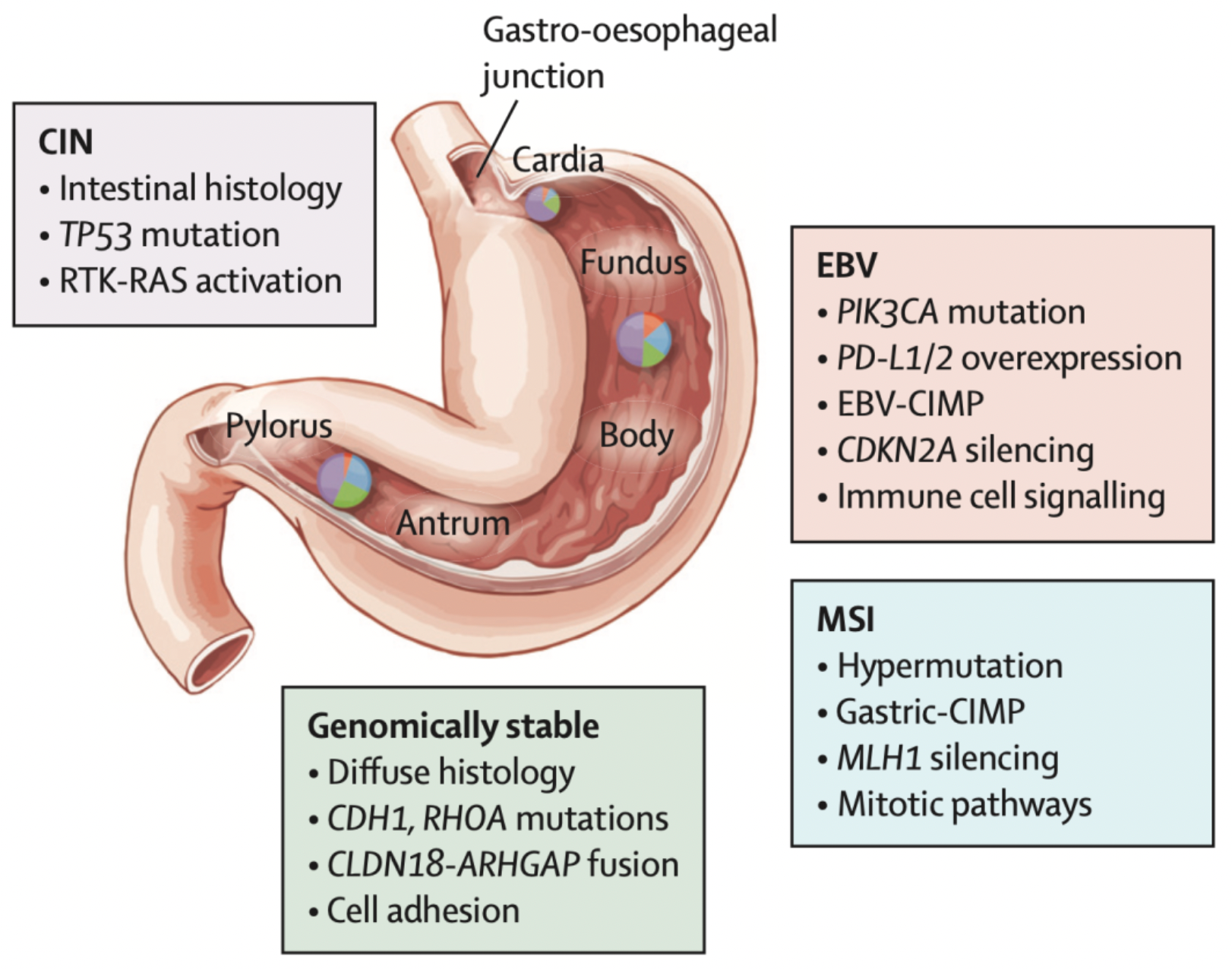
Gastric cancer can be roughly divided into two major anatomical sub-sites: although the majority of gastric cancers occur in the distal part of the stomach (non-cardia), globally, about 18% of gastric cancers originate in the cardia, which is the region adjacent to the gastroesophageal junction. Both anatomical locations of gastric cancer have overlapping risk factors, such as smoking, heavy alcohol consumption, and consumption of pickled foods; however, they also have their distinct etiologies. Cardia gastric cancer is often associated with gastroesophageal reflux disease and obesity, while approximately 90% of non-cardia gastric cancers can be attributed to Helicobacter pylori (H. pylori) infection. This implies that prevention and treatment strategies for different types of gastric cancer may need to be distinct, especially considering the rising incidence rates in young populations and the etiological differences between cardia and non-cardia gastric cancers.
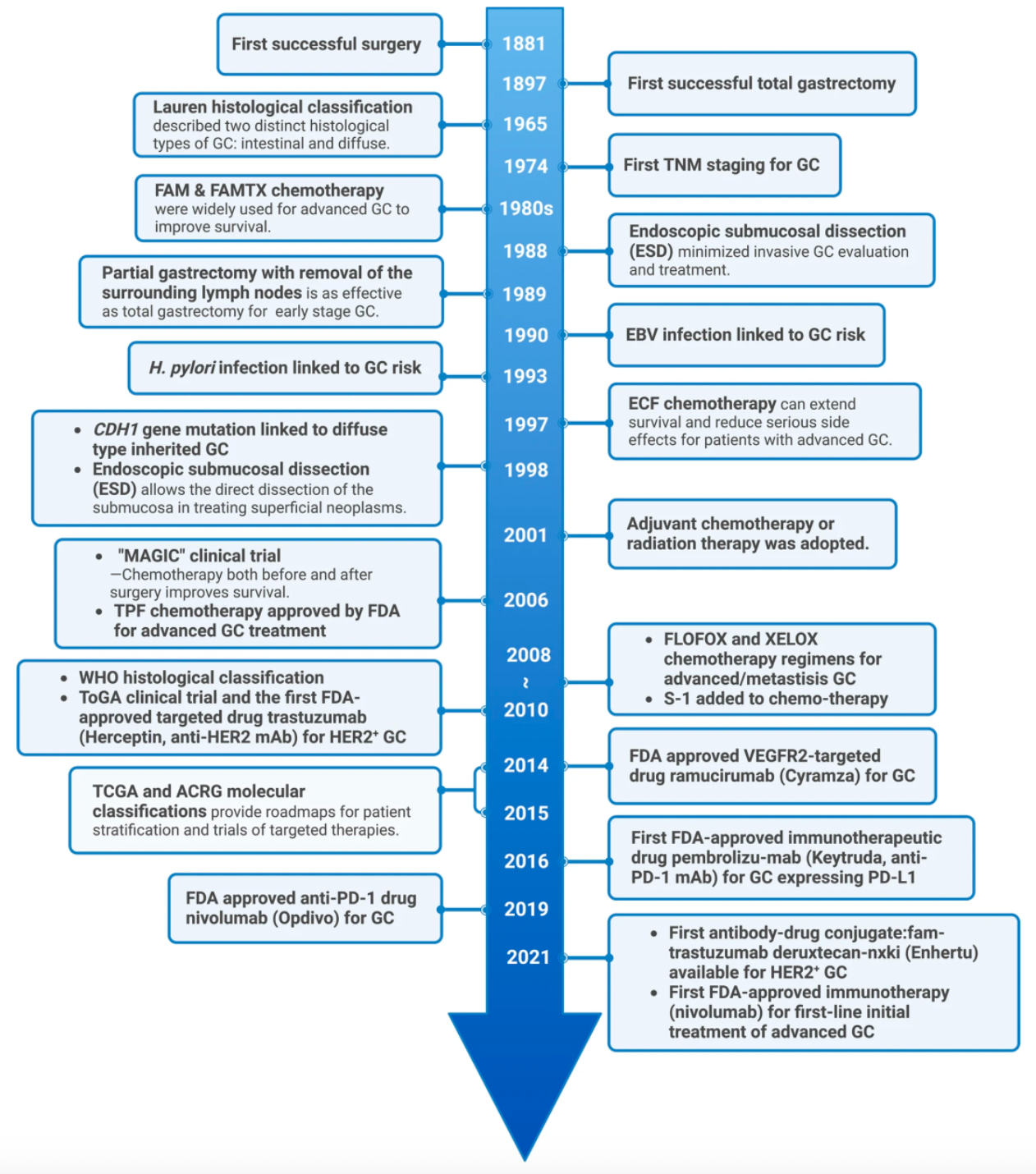
Timeline of Significant Discoveries and Key Advances in the Treatment of Gastric Cancer:
In recent years, targeted therapies against specific molecular targets have been introduced into clinical practice, such as the anti-HER2 antibody trastuzumab and the anti-VEGFR-2 antibody ramucirumab. Claudin 18.2 is also a significant target for gastric cancer treatment, as the gene is associated with specific molecular subtypes involved in the development and progression of gastric cancer. This gene and its interaction pathways serve as potential targets for the development of new personalized treatment strategies.
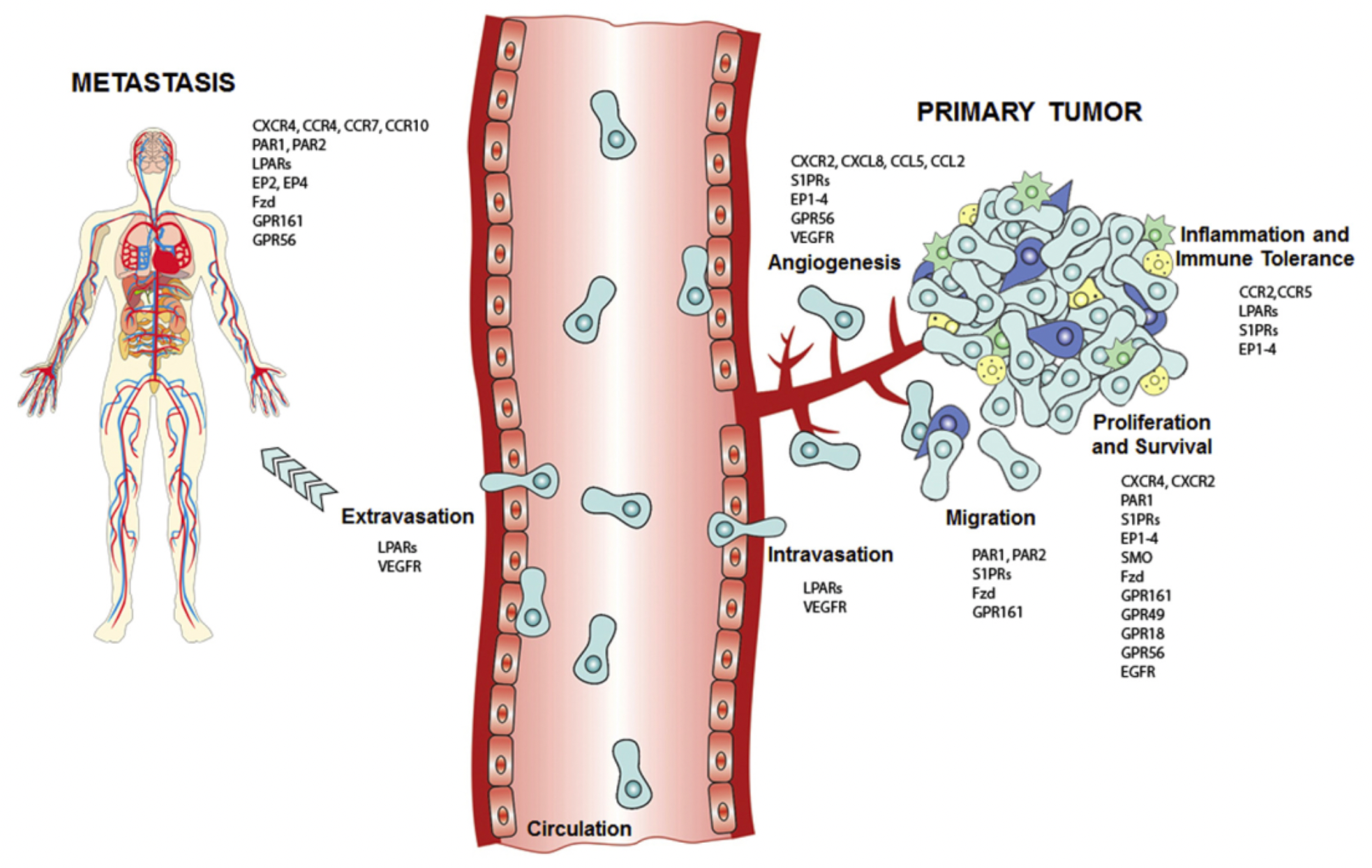
The G protein-coupled receptor (GPCR) signaling pathway is one of those pathways. Abnormal activation of GPCRs and G proteins can promote the progression of gastric cancer. Activated GPCRs/G proteins may serve as effective biomarkers for early diagnosis, prognostic prediction, and clinical therapeutic targets. This indicates that through the detection and intervention of these receptors and their aberrant activation states, it is possible to achieve early detection, accurate prognostic assessment, and targeted treatment of gastric cancer, thereby improving patient survival rates and quality of life.
GPCR and Gastric Cancer
As early as 1986, a study by Young et al. was the first to link G protein-coupled receptors (GPCRs) with tumorigenic activity. They demonstrated through experiments that the Mas proto-oncogene could confer tumorigenicity to NIH 3T3 cells in vivo in nude mice. Mas encodes a typical GPCR and, unlike most oncogenes, does not carry activating oncogenic mutations. Instead, tumorigenicity is caused by its overexpression and the abundance of ligands in the circulation system or locally produced ligands.
There is now a substantial body of evidence suggesting that various wild-type GPCRs, acting under the influence of excessive circulating agonists or agonists produced within the tumor microenvironment, can induce oncogenic transformation and drive tumor progression. Specific neuropeptides, particularly neurotransmitters and intestinal hormones, play an autocrine and paracrine role in various cancers. These neuropeptides include Gastrin-Releasing Peptide (GRP), Endothelin, Bradykinin, Neuromedin B, Neuropeptide Y, Gastrin, Cholecystokinin, Arginine Vasopressin, and Angiotensin II, among others. Each, upon binding to their respective GPCRs, triggers sustained activation, thereby stimulating proliferation in a variety of cell types and playing a crucial role in many malignant human cancers, such as small cell lung carcinoma, pancreatic cancer, head and neck squamous cell carcinoma, prostate cancer, and gastric cancer.
Progress in the development of new drugs for gastric cancer targeting GPCR-class receptors
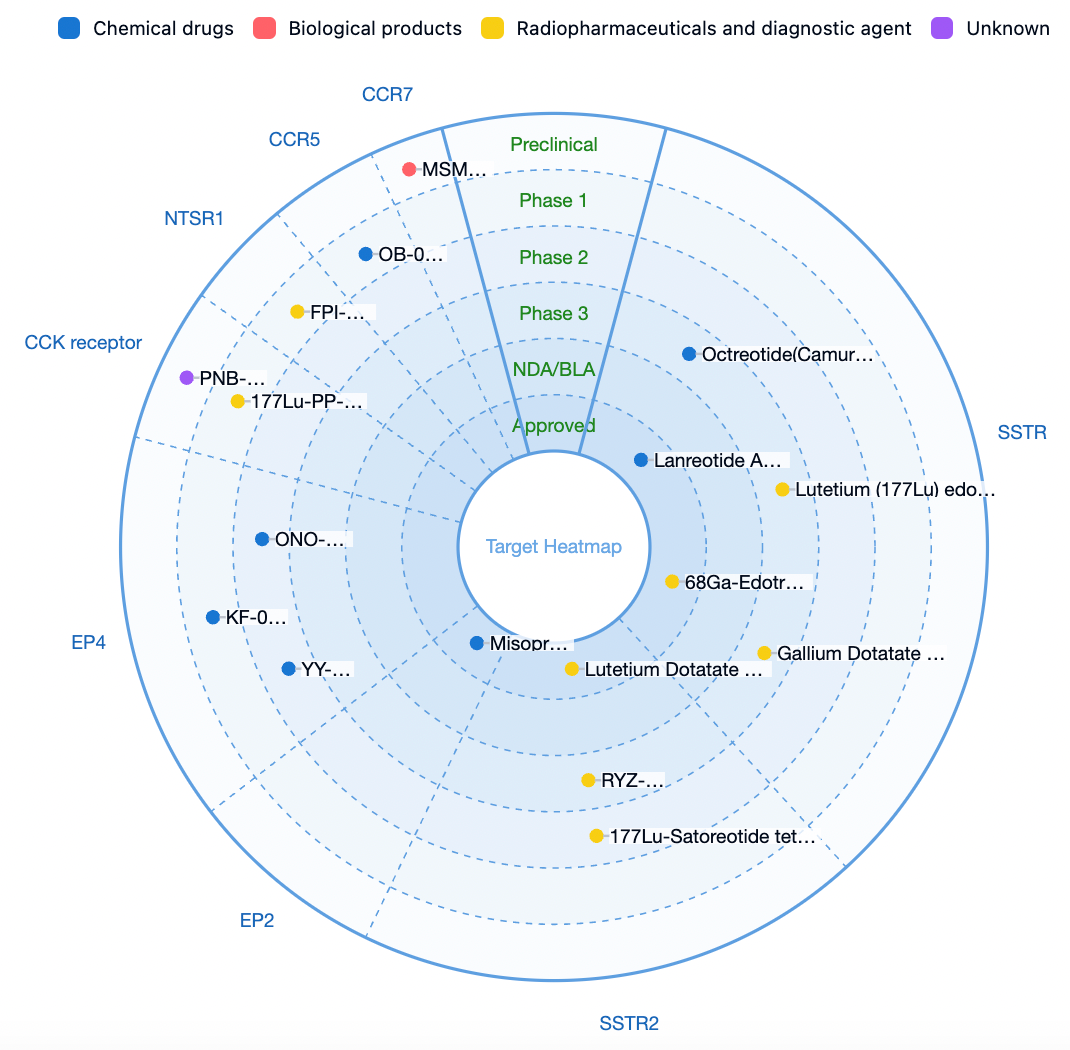
According to statistics from the Synapse database, there are currently nearly 1000 research and development pipelines for gastric cancer globally, of which 38 are GPCR (G protein-coupled receptor) type target pipelines. Popular pipeline targets include SSTR, EP2R, EP4R, CCR8, CCKR, SMO, NTSR1, A2bR, CCR7, among others.
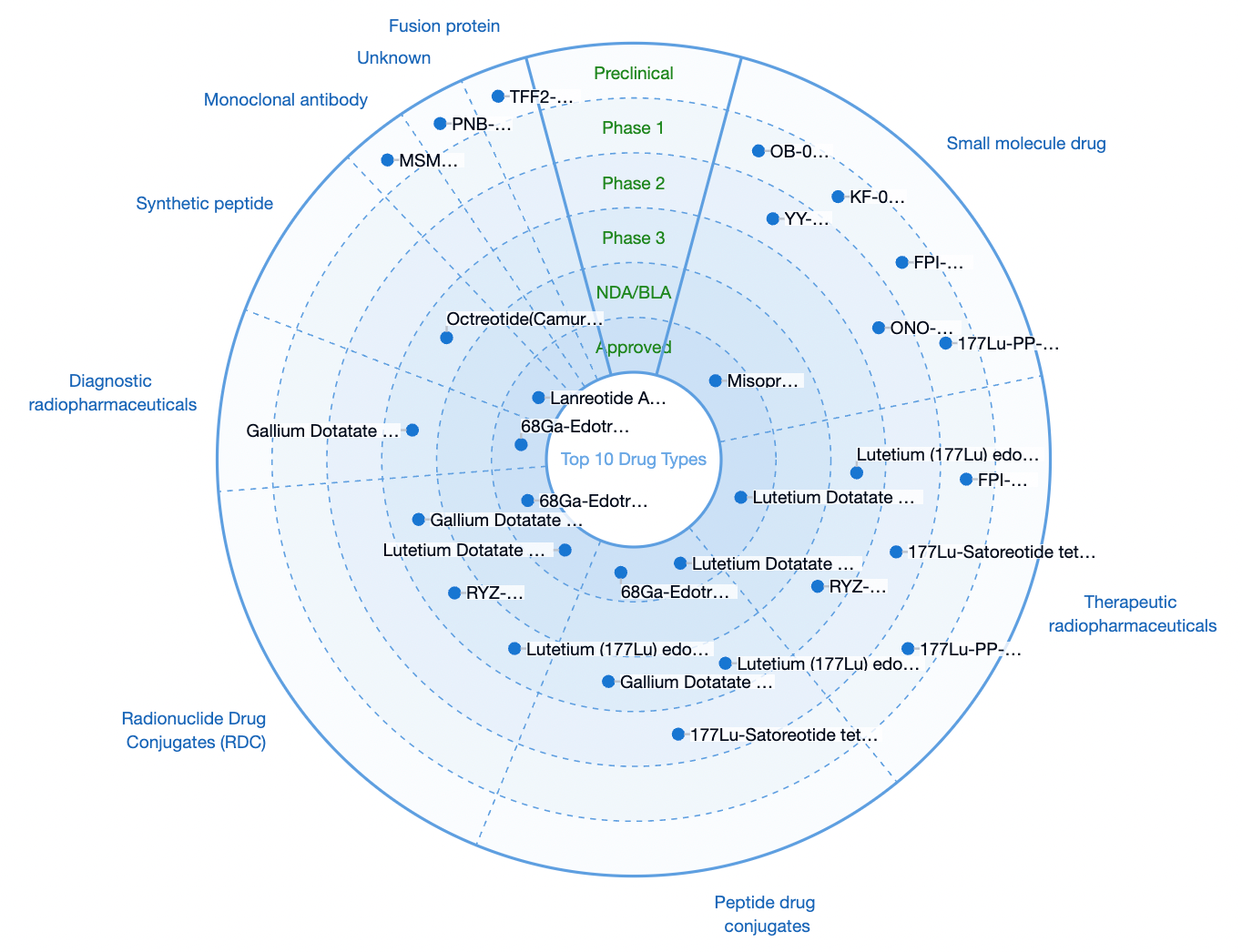
The leading indications related to gastric cancer include gastric cancer, gastro-enteropancreatic neuroendocrine tumors, gastroesophageal junction tumors, and Her2-negative gastric cancer, among others. The principal types of therapeutic agents include radiopharmaceuticals, peptide conjugates, radionuclide-conjugated drugs, synthetic peptides, monoclonal antibodies, and fusion proteins.
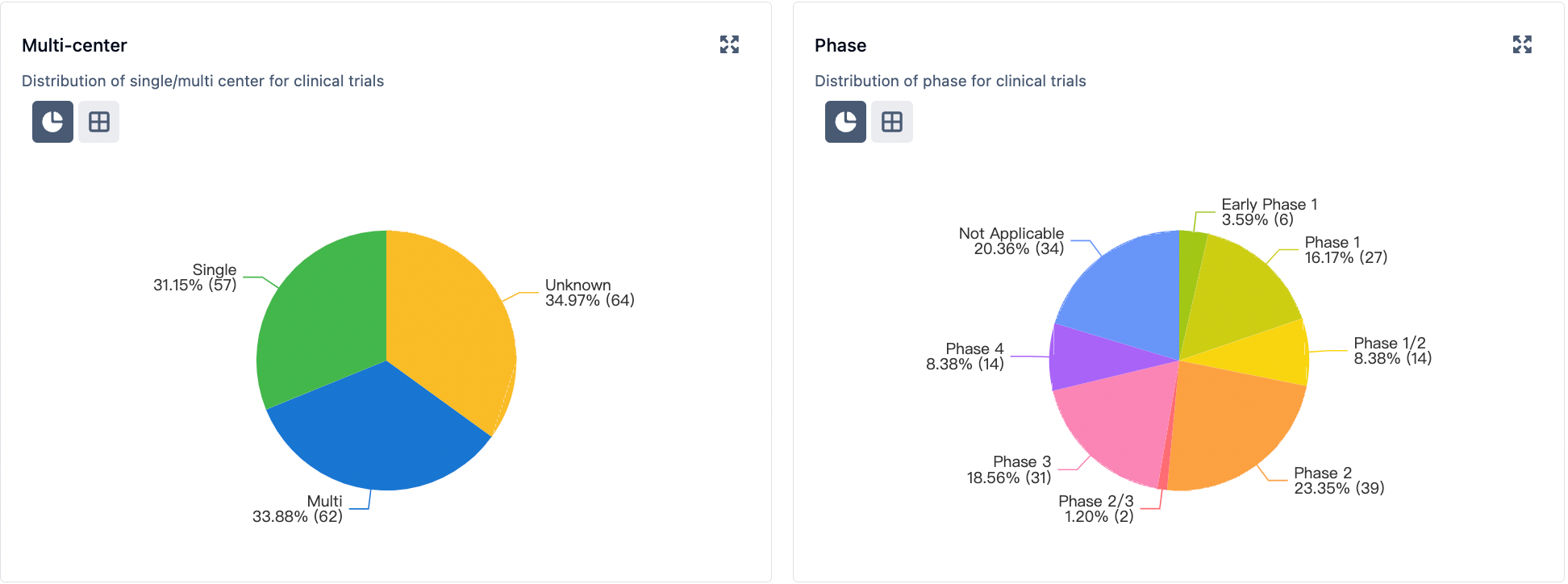
In the field of clinical research, the major regions for clinical studies on gastric medications are the United States, China, Japan, Europe, Australia, and others. Several drugs are currently in the late stages of clinical trials (Phase 2, Phase 2/3, Phase 3, etc.).
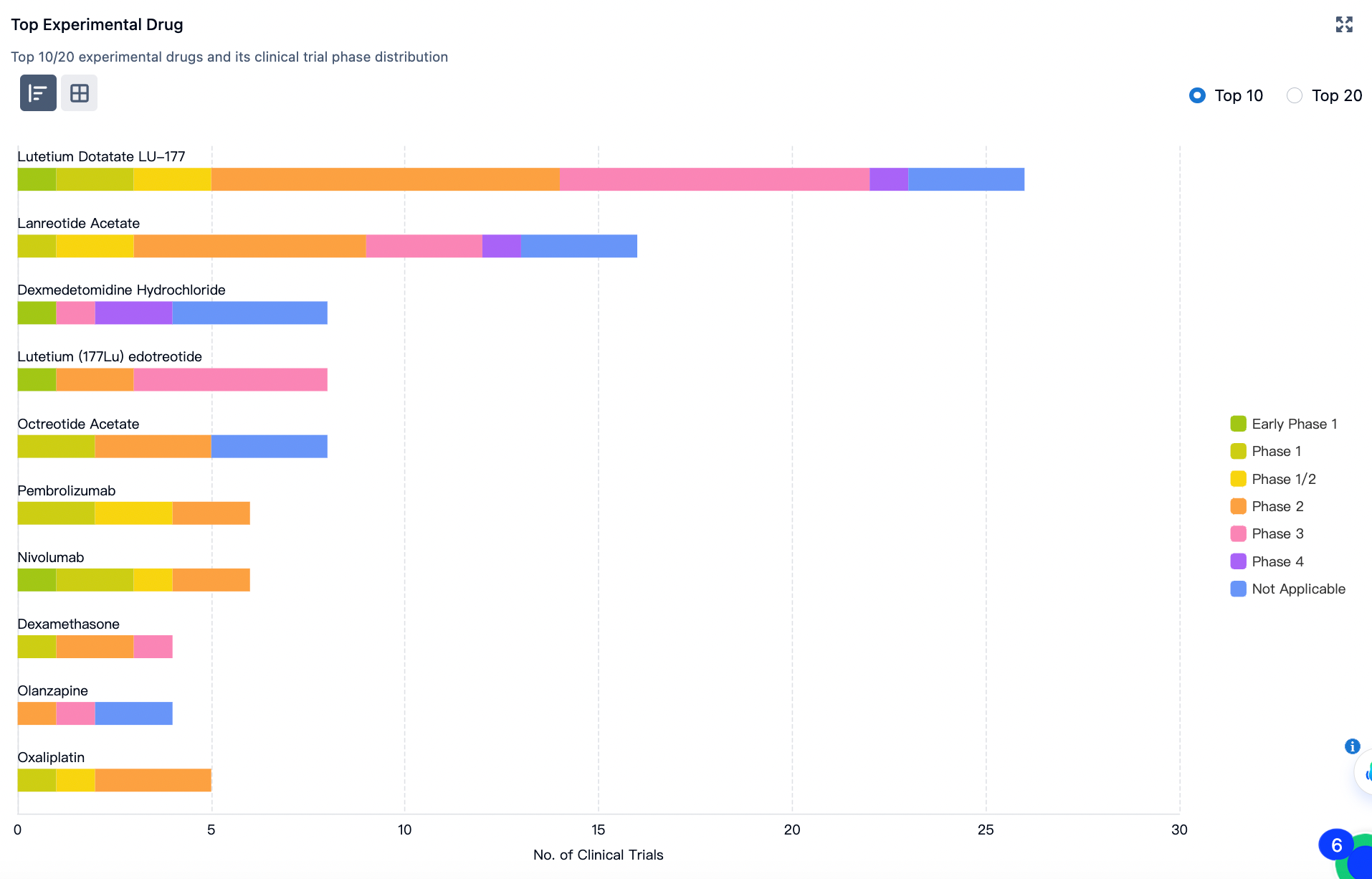
Representative investigational drugs include:
Representative Pipeline: (177Lu) Lutetium-DOTATATE and SSTRs Receptor
Lutetium-177 Dotatate (LU-177 Lutetium Dotatate, also known as Lutathera) is a radiopharmaceutical developed by Advanced Accelerator Applications. It is primarily used for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
This medicinal product is formed by the conjugation of the somatostatin analogue Dotatate with the radioactive isotope Lutetium-177. Dotatate is a compound that can specifically bind to somatostatin receptors (SSTRs) that are overexpressed on neuroendocrine tumor cells. Lutetium-177 is a beta-emitting radioisotope. When combined with Dotatate, the resultant Lutetium-177 Dotatate can accurately target and accumulate within tumor cells that express high levels of somatostatin receptors. The radioactive decay of Lutetium-177 releases beta radiation, which destroys the cancer cells, thereby achieving a localized radiation therapy effect.
Somatostatin is a peptide hormone composed of 14 amino acid residues and features a single disulfide bond. This hormone is capable of inhibiting the secretion of growth hormone, insulin, glucagon, gastrin, cholecystokinin, and thyrotropin, but due to its extremely short half-life in the body (less than 3 minutes), its value in therapy is limited. Research has identified the β-turn pharmacophore of somatostatin, which has become the lead sequence for the development of analogs with greater biological stability and potency. Based on this, octreotide was developed and approved for the treatment of acromegaly, adenomas, and pancreatic, breast, and prostate tumors. Subsequent research has spurred the development of selective receptor antagonists, such as lanreotide, vapreotide, and pasireotide, and has introduced the fields of peptide imaging and peptide receptor radionuclide therapy. In these radionuclide therapies, radioactive elements such as ^111In, ^90Y, ^68Ga, or ^99mTc are linked to selective somatostatin analogs through chelating groups, leading to approved radiopharmaceutical formulations. These drugs can be used for tumor detection.
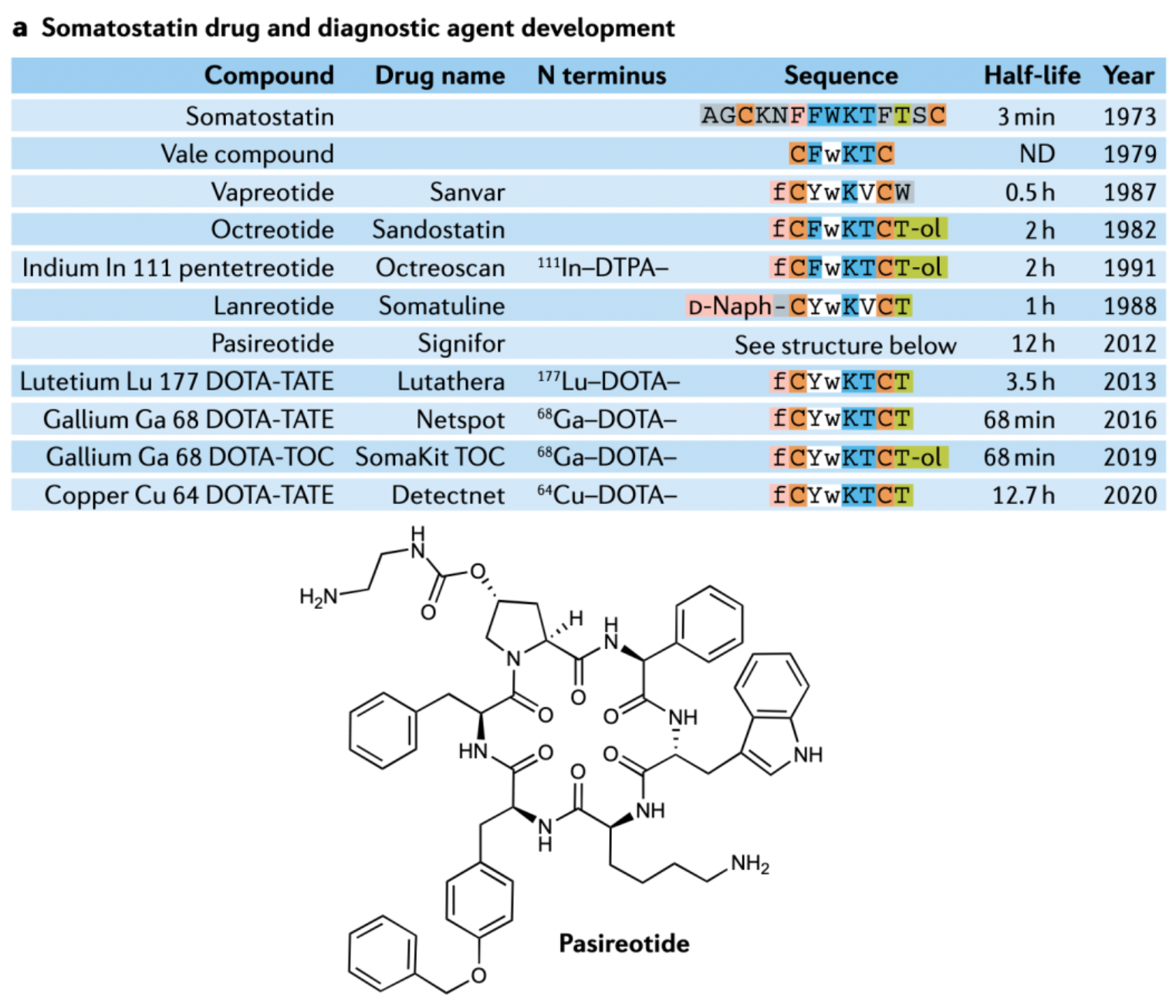
In 2001, a preclinical animal model study on Lutetium Dotatate LU-177 was published in the International Journal of Cancer. It reported on the investigation of the biologic distribution of the drug in a rat model of pancreatic tumors, and these data were used to estimate the absorbed doses in normal human tissue.
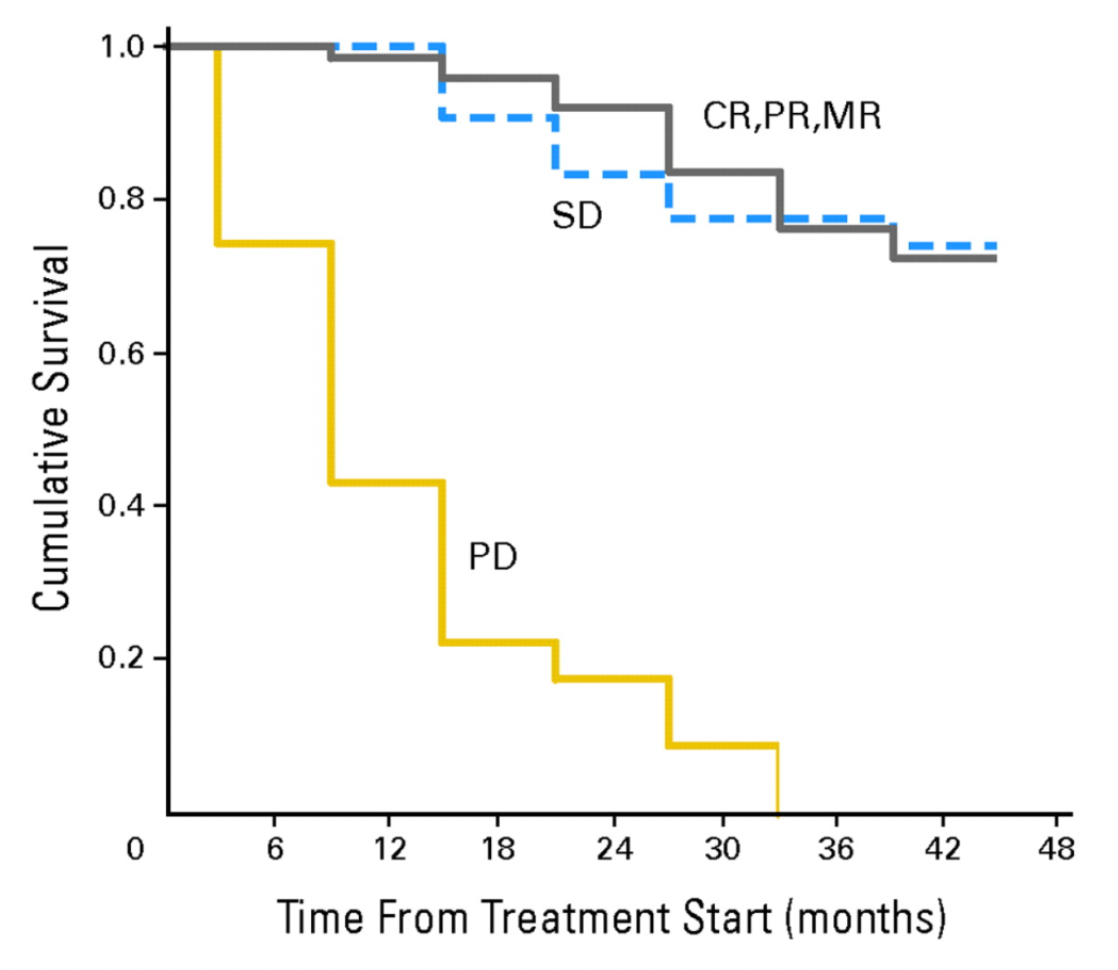
A 2004 study on the treatment effectiveness in patients with metastatic gastroenteropancreatic tumors positive for the somatostatin receptor revealed that, out of 50 patients treated with Lutetium Dotatate LU-177, disease stabilization was achieved in 24 patients, 19 patients experienced stable disease, the condition of 6 patients progressed, and 1 patient's disease status could not be assessed. The therapy significantly improved their overall health condition, quality of life, and scores for various functions and symptoms.
In 2005, a clinical study on the use of Lutetium Dotatate LU-177 in 131 patients with metastatic or inoperable somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors was announced. After treatment with a maximum cumulative dose of 600 to 800 mCi of Lutetium Dotatate LU-177, 3 patients (2%) achieved complete remission, 32 patients (26%) had partial remission, 24 patients (19%) showed a minor response (tumor size reduction of 25% to 50%), 44 patients (35%) had stable disease, whereas 22 patients (18%) experienced disease progression. A high remission rate correlated positively with high uptake shown on pre-treatment somatostatin receptor imaging and fewer liver metastases. In contrast, patients with lower performance scores and extensive disease distribution had a significantly higher frequency of disease progression. Severe side effects were very rare, and the median time to disease progression was comparable to that with chemotherapy, with better effectiveness in patients with a limited tumor burden.
An expanded clinical study published in 2008 further validated the safety and efficacy of Lutetium Dotatate LU-177. In a study involving 310 patients with gastroenteropancreatic neuroendocrine tumors, complete and partial tumor response rates were observed at 2% and 28%, respectively, with minor response rates (tumor size reduction of over 25% but less than 50%) at 16%. Compared with historical controls, this treatment method provided a survival benefit of 40 to 72 months from the point of diagnosis.
In 2017, results from a Phase 3 clinical study published in The New England Journal of Medicine indicated that, in a randomized, controlled trial involving 210 patients with gastroenteropancreatic neuroendocrine tumors, the experimental group (116 patients) receiving intravenous injections of 7.4 GBq dose of Lutetium Lu 177 Dotatate every 8 weeks for a total of four times, along with a concurrent intramuscular injection of 30 mg of long-acting octreotide every 4 weeks, had a significantly higher estimated progression-free survival rate at 20 months (65.2%) compared to the control group (10.8%), which consisted of 113 patients treated only with intramuscular injections of 60 mg of long-acting octreotide every 4 weeks. Additionally, the response rate for the Lutetium Lu 177 Dotatate group was 18%, markedly higher than the 3% observed in the control group.
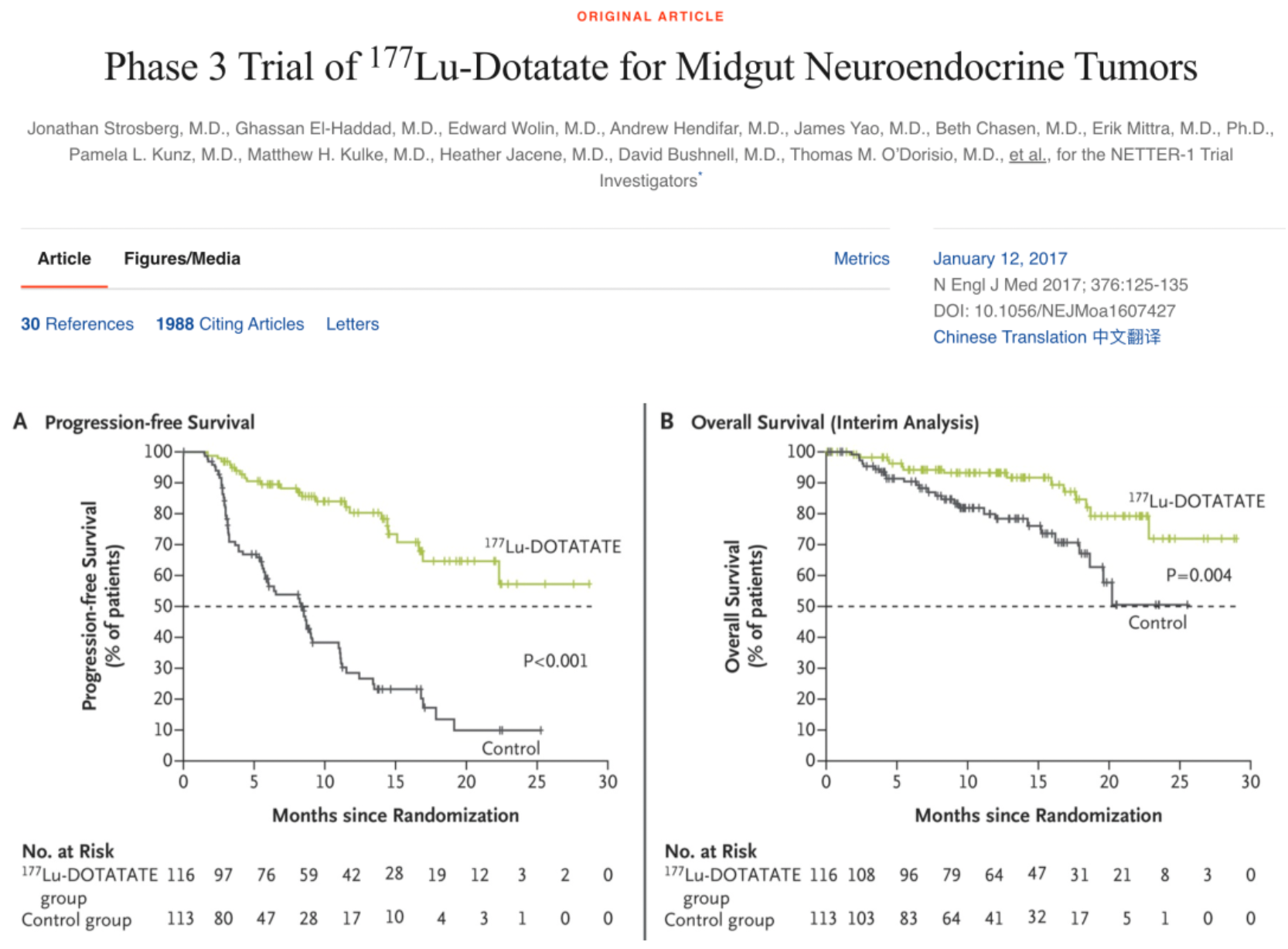
Based on this, at the end of 2017, the U.S. FDA approved Lutetium Dotatate LU-177 for the treatment of patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors. Due to its targeted nature and relatively low systemic toxicity, this therapy has received widespread attention and application in the field of precision medicine.
In October 2017, Novartis acquired the French innovative pharmaceutical company Advanced Accelerator Applications for $3.9 billion to build and expand its radioligand therapy platform. In 2018, Novartis invested an additional $2.1 billion to acquire the biopharmaceutical company Endocyte, securing several more radiopharmaceutical candidates.
Through these acquisitions, Novartis has established and extended its radioligand therapy platform, comprising multiple marketed products: Lutathera (177Lu-DOTATATE) and 68Ga-Edotreotide, as well as several products in development: 177Lu-PSMA-617, 177Lu-PSMA-R2, 177Lu-NeoB, 177Lu-FF58, 225Ac-PSMA-617, etc.
Furthermore, Lanreotide Acetate is a non-selective somatostatin receptor (SSTRs) antagonist that was first approved for marketing in 2002 for the treatment of acromegaly. Its investigational indications include gastroenteropancreatic neuroendocrine tumors.
68Ga-Edotreotide is a non-selective SSTRs antagonist product developed by Advanced Accelerator Applications. It is a radioactive diagnostic agent used in PET scans to detect somatostatin receptor-positive neuroendocrine tumors in both adult and pediatric patients.
Other related R&D pipelines
Misoprostol is a medication primarily used to reduce the risk of stomach ulcers in users of non-steroidal anti-inflammatory drugs (NSAIDs). Its mechanism of action involves stimulating prostaglandin receptors in the stomach, enhancing gastric protection against acidic substances, and reducing gastric acid secretion, thereby protecting the gastric mucosa from damage.
Additionally, Misoprostol is used for various medical applications: in the field of gynecology, it can be used to manage early miscarriage, prevent postpartum hemorrhage, and perform abortion surgery during early pregnancy. When Misoprostol acts on prostaglandin receptors in the uterus and cervix, it increases the strength and frequency of uterine contractions and decreases cervical tension, thus playing a key role in the management of miscarriage.
ONO-4578, developed by Ono Pharmaceutical, is a highly selective antagonist of the EP4 receptor and is currently being studied for its safety and efficacy as monotherapy and in combination with nivolumab in patients with advanced or metastatic solid tumors, including gastric cancer. Based on the results of a Phase 1 clinical study, ONO-4578 has demonstrated good tolerability and certain antitumor activity.
YY-001 and HTL-0039732 are selective EP4 receptor antagonists developed by Shanghai Yu Yao and Sosei Group, respectively, and are currently involved in clinical studies for various solid tumors, including gastric cancer.
BMS-986340 is an IgG1 subtype monoclonal antibody product targeting CCR8, originally researched and developed by BMS. This drug possesses enhanced non-fucosylated (NF) Fc region features. As the CCR8 receptor is a chemokine receptor expressed on the surface of regulatory T cells (Tregs) and participates in the regulation of the immune system, BMS-986340 has been engineered to specifically bind CCR8. Due to its non-fucosylated Fc segment, it enhances effector functions against target cells. This optimization enables BMS-986340 to effectively deplete regulatory T cells (Tregs), which may modulate the immune response for treating diseases associated with excessive immunosuppression or dysfunctional Tregs. Currently, clinical trials for various solid tumors, including gastric cancer, are underway involving BMS-986340.
FPI-2059 is a small molecule radiopharmaceutical that targets the Neurotensin Receptor 1 (NTSR1). This receptor is overexpressed in a variety of solid tumors, including pancreatic ductal adenocarcinoma, colorectal cancer, head and neck squamous cell carcinoma, gastric cancer, Ewing sarcoma, and neuroendocrine-differentiated prostate cancer. FPI-2059 is developed based on compounds previously known as IPN-1087 and 3BP-227. These compounds have been studied as beta-emitting radiopharmaceuticals in researcher-initiated studies and phase I clinical trials. In 2021, Fusion Pharmaceuticals acquired this asset and transformed it into an alpha-emitting radiopharmaceutical, labeled with Actinium-225. Concurrently, FPI-2058 is a diagnostic analog of FPI-2059, replacing Actinium-225 with Indium-111 as the radioactive isotope for medical imaging and diagnostic purposes. This targeted radiopharmaceutical design aims to achieve precise targeted therapy and diagnosis for the aforementioned types of tumors.
TT-4 is a selective A2B receptor antagonist developed by Tarus Therapeutics that is currently in phase 1/2 clinical development. The trial aims to evaluate its therapeutic effect in patients with various advanced solid tumors, including gastric cancer.
177Lu-PP-F11N is a radioactive drug complex that combines PP-F11N (a gastrin analog) with the beta-emitting radioactive isotope Lutetium-177. This drug exhibits potential antitumor activity and may be used as an agent for single-photon emission computerized tomography (SPECT) imaging. After intravenous injection, PP-F11N specifically binds to the Cholecystokinin-2 Receptor (CCK2R). This allows visualization of the tumor cells expressing the CCK-2 receptor using nuclear medicine imaging techniques. Additionally, the Lutetium-177 radioactive isotope delivers cytotoxic doses of beta-radiation to the tumor cells expressing the CCK-2 receptor, providing targeted destruction of these cells. As the CCK-2 receptor is widely expressed in various types of tumor cells, 177Lu-PP-F11N has the potential for the treatment of these specific types of tumors and offers targeted imaging for personalized, precise cancer diagnosis and therapy.