Immunity Pharma Reports Encouraging Initial Findings in Phase 2a Study of IPL344 Treatment for ALS
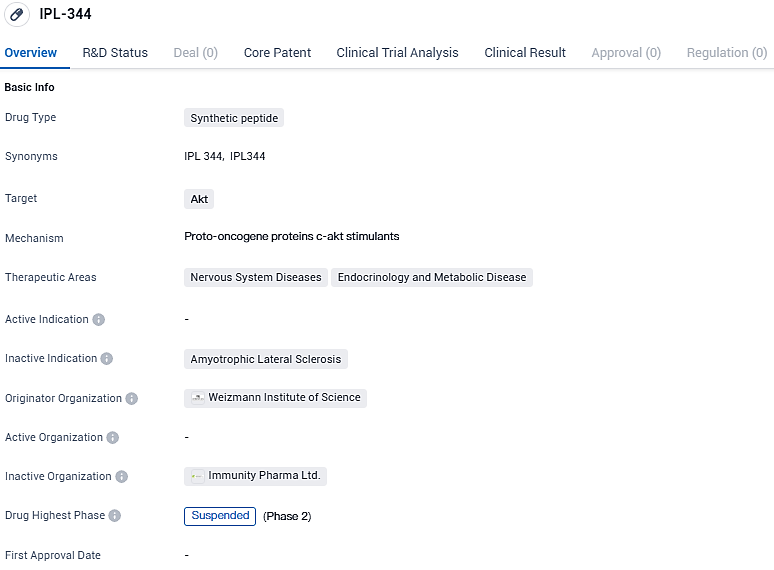
Immunity Pharma, which is currently in the clinical-stage and specializes in neurology, has revealed encouraging preliminary outcomes from its Phase 2a IPL344 test on patients with amyotrophic lateral sclerosis. The data showed a notable positive effect as signaled by a shift in the downward slope of ALSFRS-R. The medication was easily accepted and the research points to advantages in weight increase, breathing improvement, and survival. Treatment with IPL344 indicated a decrease in Neurofilament.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
During the course of this research, patients were subjected to IPL344 on a daily routine for a duration of 36 months, which was generally well-accepted with no severe side effects associated with the medication. Absolutely no participants ceased their drug treatment due to any adverse events (AEs) related to IPL344. Those patients that were treated with this drug exhibited an average ALSFRS-R decline slope of -0.53, signifying a 48% slower progress of the illness (p=0.028).
Adjustments for the state of the disease and factors indicating its rate inferred a 64% slower progression in ALSFRS-R (p=0.034). Moreover, we observed a statistically significant rise rather than a drop in the body weight of the patients treated with IPL344 (p=0.02; when compared to patients in the PRO-ACT database on placebo).
The estimated average survival duration of the patients in this research was 29 months, compared with 19 months for placebo-treated patients in the ceftriaxone research, suggesting a potential decrease in mortality rate favoring IPL344 (p=0.13). The study also revealed a non-significant tendency to maintain respiratory capacity (44% slower reduction in average %SVC loss on a monthly basis vs historical placebo; p=0.15).
"We are thrilled that IPL344 treatment had a good tolerability profile among patients suffering from ALS and showed positive indicators of working effectively in several aspects. We plan on putting this collected data forward for publication in a science-based publication," stated Dr. Ilana Cohen, the VP of R&D at Immunity Pharma. "If these initial findings are verified in a subsequent enlarged phase 3 research, the degree of reduction in ALSFRS-R progression in this research surpasses that of the drugs currently approved."
"These initial findings validate the continued development of IPL344 as a potential therapy for ALS, and justifies a more extensive investigation involving many more patients. Our aim is to take IPL344 to a decisive clinical research in ALS," conveyed Immunity's CEO, Eran Ovadia.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
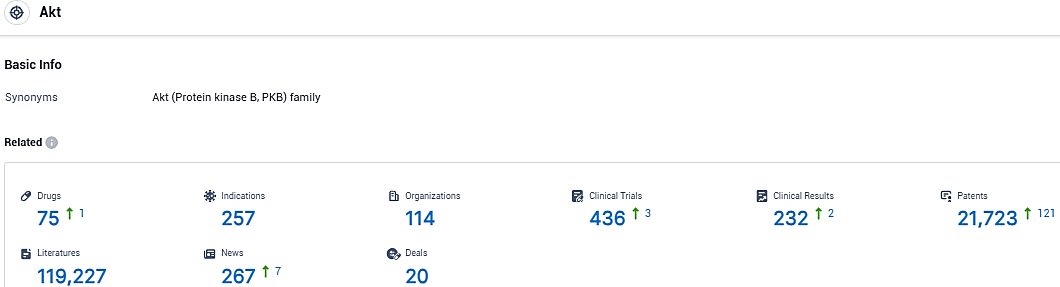
According to the data provided by the Synapse Database, As of January 24, 2024, there are 75 investigational drugs for the Akt target, including 257 indications, 114 R&D institutions involved, with related clinical trials reaching 436, and as many as 21723 patents.
IPL344 is a biologically active peptide that stimulates therapeutic cell-signaling processes including activation of the Akt pathway, which is down-regulated in neurodegenerative diseases. IPL344 was discovered in the Weizmann institute of Science, Israel, at Prof. Irun Cohen's Laboratory. IPL344 is being developed as an intravenous injection for the treatment of ALS initially through a Phase 1/2a clinical trial in ALS patients. IPL344 received orphan drug designation from FDA and EMA, which grants exclusivity for at least seven years.