Adagene's ADG126 (muzastotug) and Pembrolizumab Show Superior Traits for Advanced MSS CRC Treatment
Adagene Inc., an innovator in the field of antibody-based therapeutic research and development, publicized findings during their session at the 2024 American Society of Clinical Oncology's Symposium on Gastrointestinal Cancers. The event occurred from the 18th to the 20th of January in San Francisco.
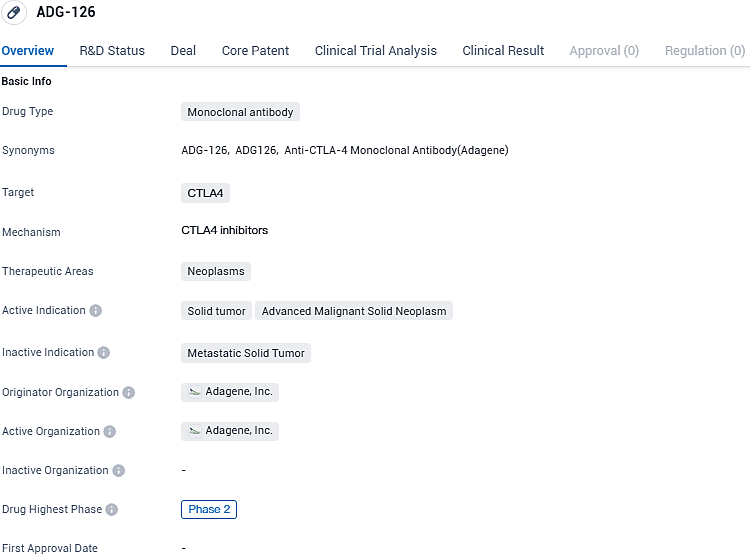
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Enhanced dosing regimens for ADG126, a novel form of CTLA-4 targeted therapy, are now achievable, increasing both the dose and frequency over existing treatments," reported Daneng Li, MD, who is an Associate Professor in the Department of Medical Oncology & Therapeutics Research at City of Hope Comprehensive Cancer Center.
Dr. Li added, "The encouraging findings from recent studies suggest we should proceed with more in-depth investigations of ADG126, an anti-CTLA-4 antibody that could potentially lead its class, particularly in association with pembrolizumab for MSS CRC sufferers. This includes efforts to confront emerging liver lesions in patients who initially show no signs of liver metastases. There is also an exciting possibility of extending its use to a variety of other cancer types in need of more effective and safer anti-CTLA-4 interventions."
Heinz Josef-Lenz, MD, FACP, the Associate Director for Clinical Research and a Senior Member of the Translational Science Program at USC Norris Comprehensive Cancer Center, offered his insight on the matter: "Targeting CTLA-4 is crucial to achieve the desired outcomes in immunotherapy treatments for MSS CRC, yet current first-generation treatments have been hindered by their safety profiles. The combined application of ADG126 with pembrolizumab could provide substantial benefits to MSS CRC patients, especially since these patients typically have very few immunotherapeutic alternatives at their disposal."
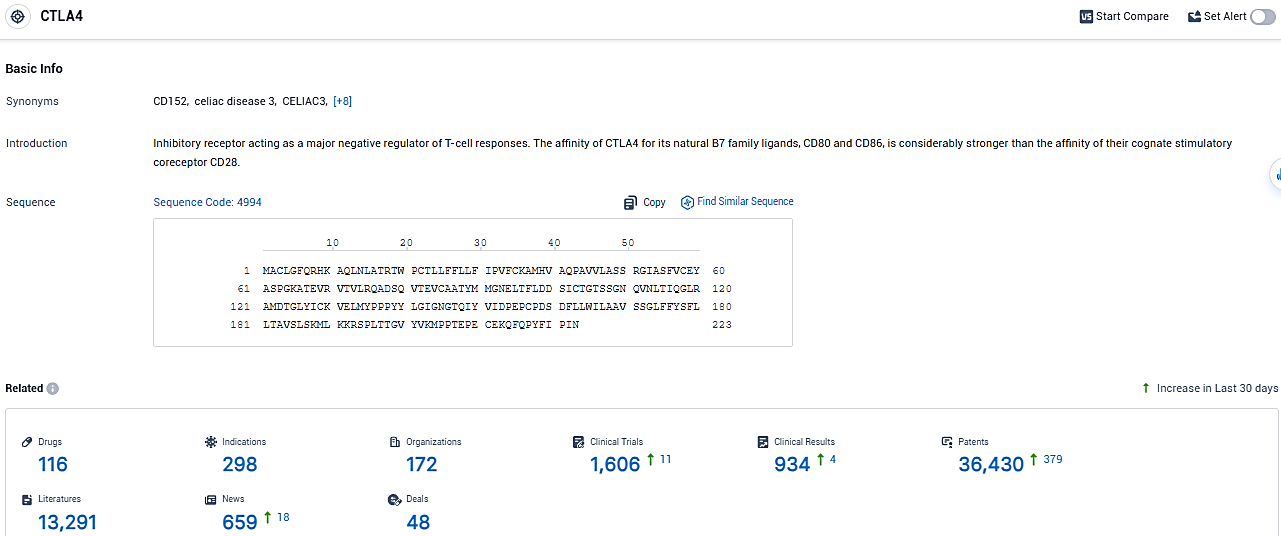
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 24, 2024, there are 116 investigational drugs for the CTLA-4 target, including 298 indications, 172 R&D institutions involved, with related clinical trials reaching 1606, and as many as 36430 patents.
ADG-126 shows promise as a potential treatment for solid tumors and advanced malignant solid neoplasms. Its specific targeting of CTLA4 suggests that it may have a role in modulating the immune response against cancer cells. However, further clinical trials are needed to determine its safety and efficacy in larger patient populations.