Positive Outcome for Henlius Phase 1 HLX14 Trial Confirmed
Shanghai Henlius Biotech, Inc. has reported that its initial clinical research, phase 1, examining their biosimilar HLX14, an injectable form of recombinant anti-RANKL human monoclonal antibody, successfully achieved the main objective of the study.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The findings from the research indicate that HLX14 demonstrated pharmacokinetic (PK) and pharmacodynamic (PD) profiles that were notably congruent with those of reference biologic medicines from varying origins, while also showing equivalent profiles in terms of safety, tolerability, and immunological response. The research achieved every one of the planned objectives. The enterprise is currently engaged in a widespread phase 3 comparative clinical trial of HLX14, targeting postmenopausal females with a heightened fracture risk due to osteoporosis, at multiple international sites.
Henlius has autonomously engineered the denosumab biosimilar HLX14, adhering to the standards set by the NMPA, EMA, FDA, and additional global biosimilar protocols. This includes a phase 1 clinical trial which is divided into two sections, involving healthy adult males from the Chinese demographic. The first section is an open-label, randomised, parallel-group, single-dose, dual-arm pilot investigation, primarily aiming to evaluate the pharmacokinetic attributes of HLX14 against EU-acquired denosumab to underpin the design of the subsequent part of the study.
In this initial part, the secondary aim is the assessment of PD, safety, tolerability, and immune response of HLX14 in comparison to EU-sourced denosumab. The subsequent part is a double-masked, randomised, parallel-group, single-dose, quadruple-arm evaluation focusing principally on the PK resemblance of HLX14 with denosumab sourced from the US, EU, and China. For Part 2, the secondary aim involves contrasting PD, safety, tolerability, and immune response among HLX14 and the standard reference biologics.
According to the study's outcomes, HLX14 shares a substantial similarity in PK and PD with reference biologics from multiple sources, indicated by the geometric mean ratios for the chief PK endpoints being close to 1, and their 90% confidence intervals entirely placed within the predefined range of equivalence, stretched from 0.8 to 1.25. The aspects of safety, tolerability, and immunogenic responses were found to be on par. The study successfully reached all the established objectives.
HLX14 is designed to selectively adhere to RANKL and obstruct the connection between RANKL and RANK, which appears on the surface of osteoclasts, subsequently hindering the RANKL/RANK driven process of differentiation, maturation, and activation of osteoclasts. This action consequently diminishes bone resorption and lowers the occurrence of bone-related adverse events.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
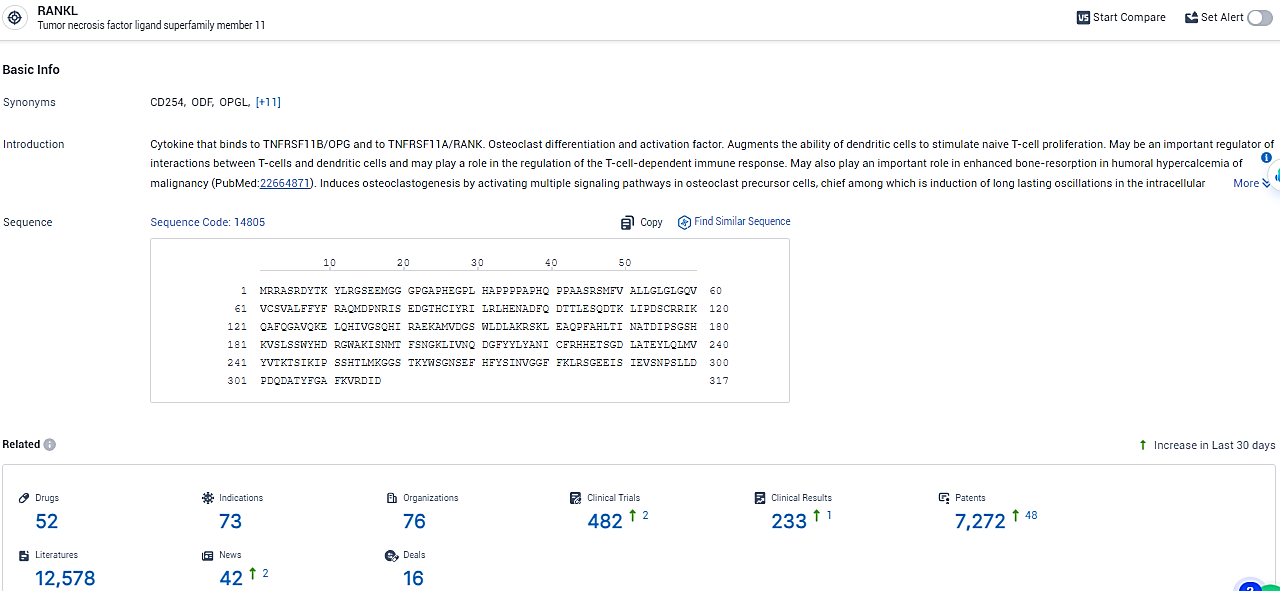
According to the data provided by the Synapse Database, As of February 8, 2024, there are 52 investigational drugs for the RANKL target, including 73 indications, 76 R&D institutions involved, with related clinical trials reaching 482, and as many as 7272 patents.
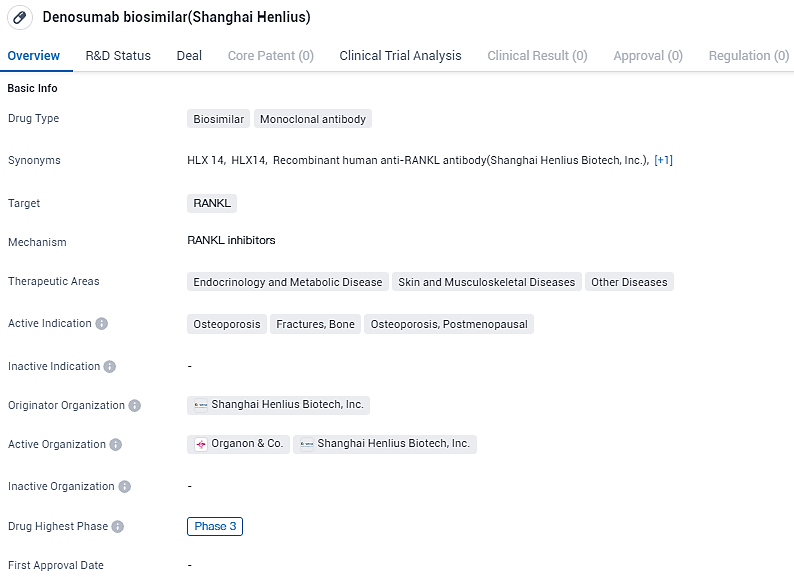
HLX14 shows promise in treating osteoporosis, fractures, and bone-related complications, particularly in postmenopausal women. With its highest phase of development being Phase 3, Denosumab biosimilar is advancing towards potential regulatory approval, offering a potential alternative treatment option in the field of biomedicine.