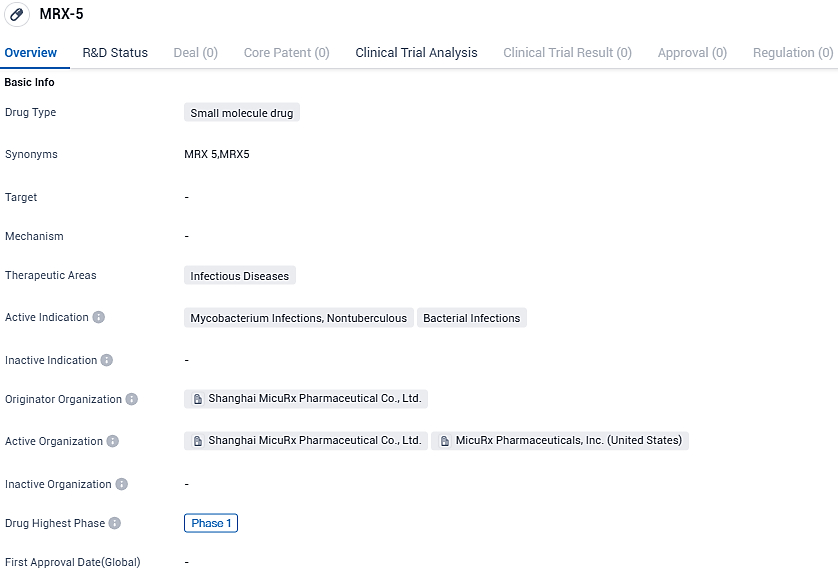
Initial Participant Registered for the Phase 1 Investigation of MRX-5
Recently, Shanghai MicuRx Pharmaceuticals Co., Ltd. revealed that the first volunteer has been enlisted for the initial Phase I clinical examination of its innovative antibiotic MRX-5 in Australia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
MRX-5 represents a novel benzodiazepine antibiotic for addressing infections triggered by NTMs (non-tuberculous mycobacteria)). This group of bacteria - excluding Mycobacteria tuberculosis - instigates chronic pulmonary infections resulting in lung deterioration and increased fatality rates. Globally, the active cases treating NTM infections outstrip 250,000 and it's proliferation is on the rise. Thus far, over 190 species of NTMs, along with 14 subspecies have been recognized, including dominantly pathogenic NTMs like Mycobacterium abscess and Mycobacterium avium complex. The managing of NTM infections entails an antibiotic regimen for 12-24 months, administered multiple times daily.
With current treatment approaches, the patient experiences a multitude of adverse reactions, lacking clinical resolution, patient non-compliance and a bleak prognosis. Particularly for resistant NTM patients, the treatment demand is not fully catered to. The healthcare field eyes the development of improved antibiotics that yield higher efficiency, reduced ill-effects and superior patient compliance.
In taking strides towards this objective, MicuRx is engaged in progressing MRX-5 to combat resistant NTM infections. Preliminary studies have indicated the robust antibacterial properties of MRX-5 against most common NTM bacteria, and its desirable safety in animal toxicology studies.
In this regard, the inaugural Phase 1 human clinical trial of MRX-5, executed at Nucleus Network Research Centre in Australia, aims at evaluating the safety, tolerance, pharmaco-dynamics, and dietary impact of orally administered MRX-5 in healthy subjects. The trial secured prior clearance from the Australian Ethics Commission for Human Research and has finalized the clinical trial submission to the Therapeutic Goods Administration.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
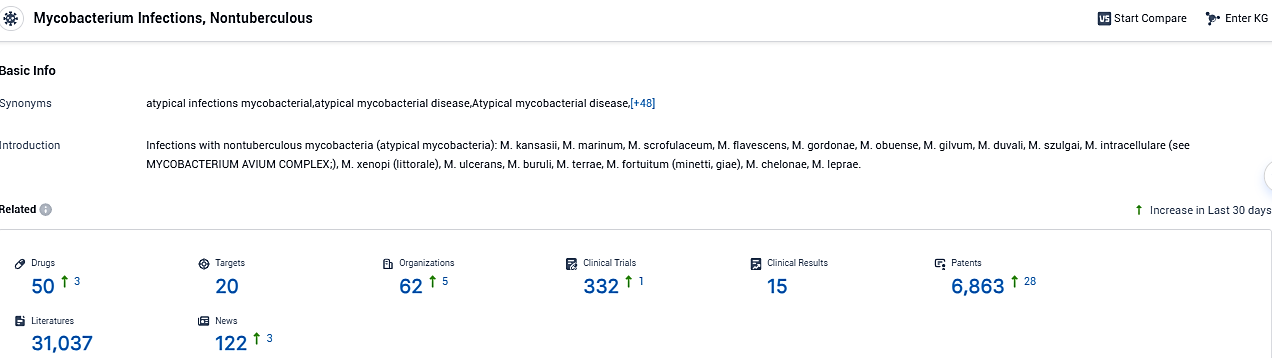
According to the data provided by the Synapse Database, As of November 28, 2023, there are 50 investigational drugs for Nontuberculous Mycobacterium Infections , including 20 targets, 62 R&D institutions involved, with related clinical trials reaching 332, and as many as 6863 patents.
MRX-5 has reached Phase 1 of clinical development globally and is currently in the preclinical stage in China. The drug holds potential in addressing the unmet medical needs in the field of infectious diseases, and further clinical trials will be necessary to evaluate its safety and efficacy.