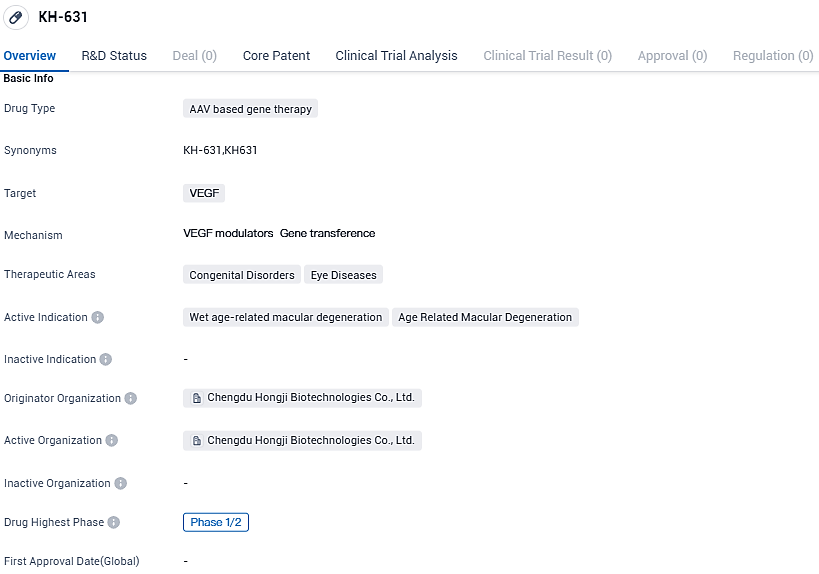
Origen Chengdu and Vanotech announce first patient treated with VAN-2201 gene therapy in Phase 1 trial for age-related Wet Macular Degeneration
The initiation of the Phase 1 clinical trial for the VAN-2201 multi-center, conducted jointly by Chengdu Origen Biotechnology Co., Ltd. and Vanotech Ltd., has been declared, signifying that the first patient has been administered with KH631 for the treatment of wet Age-related Macular Degeneration. This clinical trial, which is open-label and involves dose-escalation, will be carried out across various U.S. centers, and is aimed at determining the safety, tolerability, and effectiveness of KH631. This gene therapy will be administered once to previously treated wet AMD patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
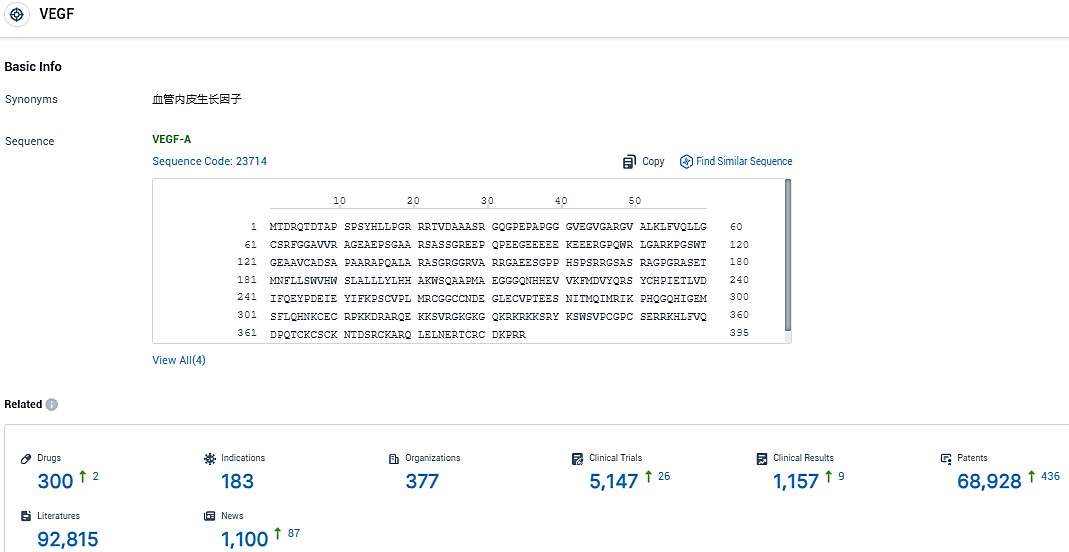
VEGF The recombinant adeno-associated virus vector, KH631, carries the genetic instructions for a human VEGF receptor fusion protein. Throughout lab-based investigations of models replicating wet AMD, the application of KH631 ensured prolonged retention of the transgene product within the retina and halted disease progression. This may suggest the capability of KH631 to function as a single-dose treatment for patients suffering from wet AMD.
"The initiation of the VAN-2201 Phase 1 trial by administering the therapy to our first patient signifies a significant stride in the progression of KH631. We are probing the viability of a single gene therapy administration as a form of treatment for wet AMD. KH631 is engineered to continuously transport anti-VEGF to the retina, possibly maintaining steady levels that can manage the disease," explained Avner Ingerman, M.D., the top medical officer at Vanotech.
"The chance to partake as a principal investigator in this pivotal investigation thrills me. This first patient dosing signifies a noteworthy progression in the development of an approach utilizing a one-time gene therapy, which could offer hope to patients needing regular treatments for their retinal conditions," said Jeffrey S. Heier, M.D., who serves as principal investigator in the VAN-2201 Phase 1 trial and holds the role of Director of the service offering Vitreoretinal services and also of Retina Research at Boston's Ophthalmic Consultants.
VAN-2201 is basically a Phase 1, multi-center study held in an open-label manner with dose-escalation, centring around patients dealing with wet AMD. The study is set on assessing the safety, tolerance, and effectiveness of a single administration of KH631. The research is projected to involve around twenty-five subjects previously treated for wet AMD who’ve shown to be responsive to anti-VEGF therapy.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 28, 2023, there are 300 investigational drugs for the VEGF target, including 183 indications, 377 R&D institutions involved, with related clinical trials reaching 5147, and as many as 68928 patents.
As KH-631 is currently in Phase 1/2 of development, further clinical trials and research are needed to evaluate its safety and efficacy. However, the use of AAV based gene therapy and the targeting of VEGF show promise in the treatment of congenital disorders and eye diseases. The progress of KH-631 in clinical development will be closely monitored to assess its potential impact on patients suffering from wet AMD and age-related macular degeneration.