Inmagene created Celexor Bio, based on its anti-ILT7 mAb displaying enhanced pDC reduction capabilities
Inmagene Biopharmaceuticals, an advanced biomedical firm striving to generate novel distinctive treatments for immunological and inflammation diseases, unveiled its collaboration with Aditum Bio to establish Celexor Bio, a fresh biotechnological entity concentrated on eradicating harmful cells in autoimmune and inflammatory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
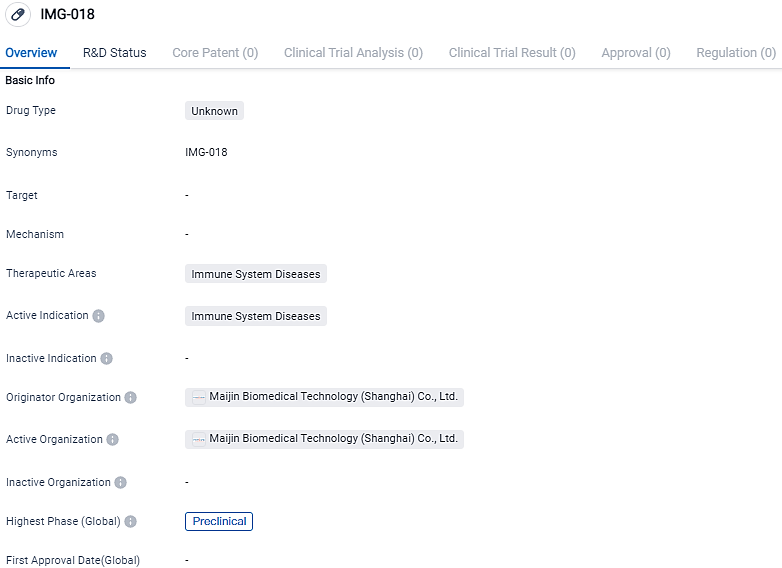
Celexor was created utilizing Inmagene's IMG-018, a pDC-depleting antibody coined to immunoglobulin-like transcript 7 (ILT7). Celexor is expected to secure exclusive worldwide rights for the development, production, and sale of IMG-018, hereafter to be known as CLXR-901, in a deal set to conclude this month.
"We're excited to collaborate with Aditum Bio, a leading-edge venture capital firm, founded by accomplished pharmaceutical market veterans," stated Jonathan Wang, PhD, Inmagene's CEO. "The conception of Celexor, based on IMG-018, showcases Inmagene's prowess in unearthing distinctive and ground-breaking drug potentials."
Joe Jimenez, Aditum Bio Co-Founder and Managing Director said, "Aditum Bio's unique approach of nurturing startups fast-tracks the evolution of new medicines. We're eager to introduce this promising therapy to patients who require it."
Under the accord's conditions, an initial payment will be given to Inmagene and they stand to gain up to $287 million via development and sales based milestones, along with royalties on future sales of the drug. Moreover, Inmagene will receive an equity interest in Celexor Bio.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, target, organizations, clinical trials, clinical results, and drug patents related to this indication.
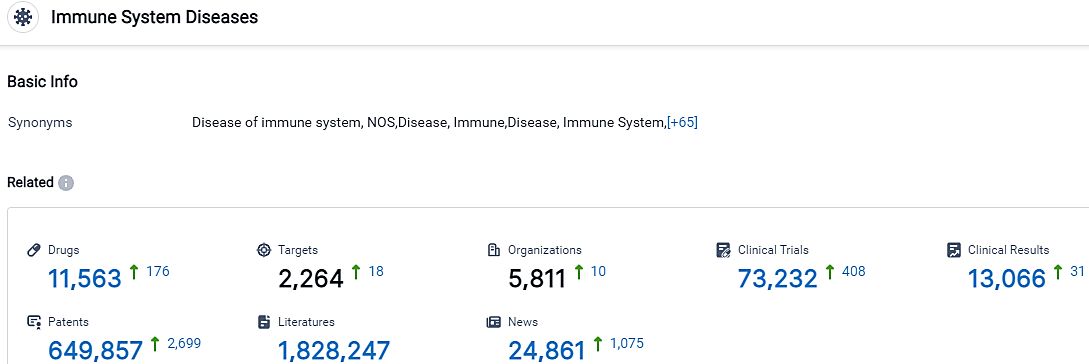
According to the data provided by the Synapse Database, As of September 26, 2023, there are 11563 investigational drugs for the Immune System Diseases, including 2264 indications,5811 R&D institutions involved, with related clinical trials reaching 73232,and as many as 649857 patents.
Engineered through Inmagene's unique QuadraTek® technology platform, IMG-018 is a type of monoclonal antibody that attaches itself to the ILT7 receptor found on the outside of plasmacytoid dendritic cells, and brings in helper cells which aid in the reduction of pDCs. These characteristics may position IMG-018/CLXR-901 uniquely for the treatment of immune ailments where the ILT7 receptor and pDCs play a significant role.