Inmagene Reports Promising Phase 2a Results for IMG-007, a Nondepleting Anti-OX40 Antibody, in Atopic Dermatitis Treatment
Inmagene Biopharmaceuticals, a biotech firm at the clinical stage focused on creating unique therapies for immune and inflammatory conditions, recently disclosed encouraging preliminary results from a Phase 2a study of IMG-007 in individuals suffering from AD.
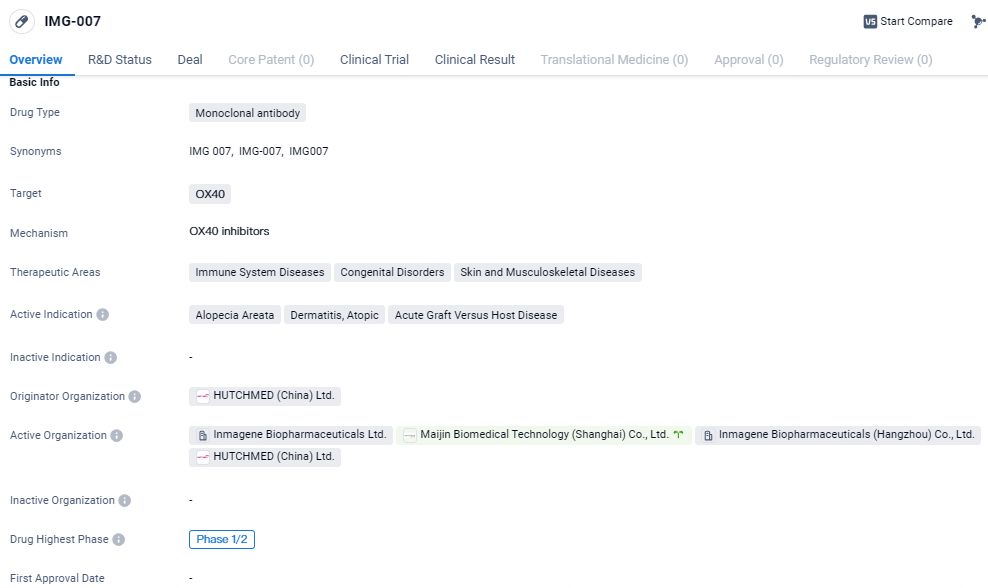
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
IMG-007, a nondepleting anti-OX40 monoclonal antibody, has been engineered to mute its antibody-dependent cellular cytotoxicity (ADCC) and extend its half-life. Previous Phase 1 research involving a single dose administered to healthy volunteers confirmed that IMG-007 was safe and well-tolerated, showing no signs of fever or chills, which aligns with the suppressed ADCC activity.
Moreover, the antibody displayed delayed elimination from the body with a half-life of 31 days at the doses expected for therapeutic use. This extended half-life facilitates less frequent administration schedules, potentially every 12 weeks for initial therapy and more sparingly for ongoing management in treating atopic dermatitis (AD).
"The interruption of OX40-OX40L interactions presents a promising method for AD management," comments Jonathan Silverberg, M.D., Ph.D., a Dermatology Professor at The George Washington University School of Medicine and Health Sciences. "IMG-007's specific targeting mechanism for the OX40 receptor, paired with an extended half-life and non-T-cell depleting qualities, offers a foremost advantage in safety and treatment convenience, including quarterly dosing."
Yufang Lu, M.D., Ph.D., Chief Medical Officer at Inmagene, shares enthusiastic remarks about the treatment's effectiveness, "The short 4-week treatment period already exhibited significant results. Ongoing and future trials using IMG-007 consistently in AD patients may enhance these outcomes even further," Lu states. "Considering the critical function of the OX40-OX40L pathway in various immune and inflammatory diseases, IMG-007 holds potential across multiple clinical conditions. Efforts continue to fast-track its development for AD using a subcutaneous approach."
Additionally, IMG-007 is under investigation in a Phase 2a study targeting AD, with final outcomes expected by Q3 2024. It is also part of a global clinical trial for adult patients with alopecia areata (AA), with preliminary data expected in Q4 2024.
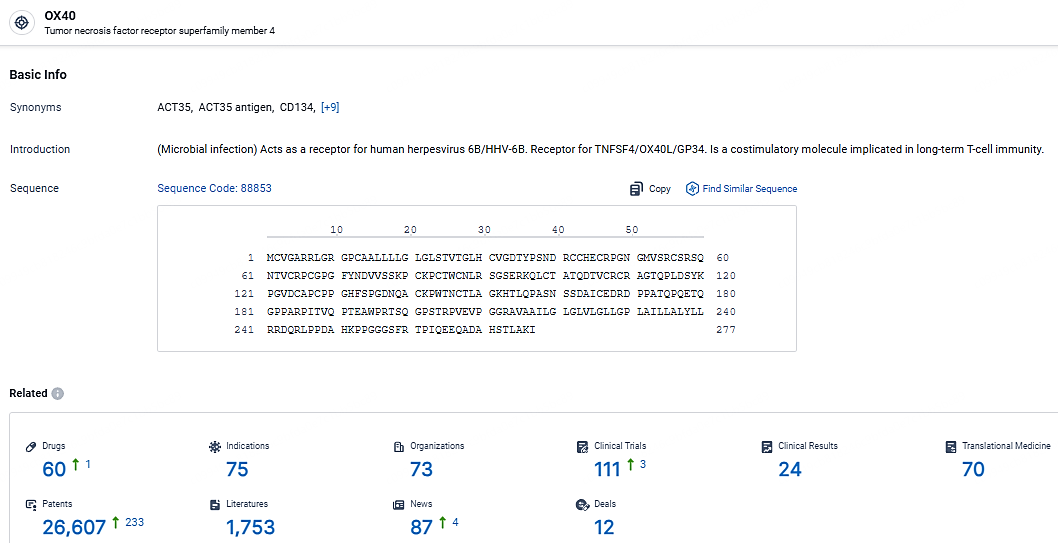
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 7, 2024, there are 60 investigational drugs for the OX40 target, including 75 indications, 73 R&D institutions involved, with related clinical trials reaching 111, and as many as 26607 patents.
IMG-007 targets OX40 and is being investigated for its potential in treating immune system diseases, congenital disorders, as well as skin and musculoskeletal diseases. Currently in Phase 1/2 of clinical development globally, IMG-007 has received IND approval in China.