Innovent Starts Phase III Neoshot Trial with IBI310 Plus Sintilimab in Colon Cancer
Innovent Biologics, Inc., has publicly declared the initiation of a new phase in their clinical research, marking a significant milestone with the administration of the initial dose to a patient enrolled in their study. This marks the beginning of the treatment phase with IBI310, an anti-CTLA-4 monoclonal antibody, which is being tested alongside sintilimab. The study design is that of a randomized, multicenter Phase 3 trial, and it is focused on investigating the efficacy of these drugs as a pre-surgical therapy for patients with MSI-H/dMMR colon cancer that can be surgically removed.
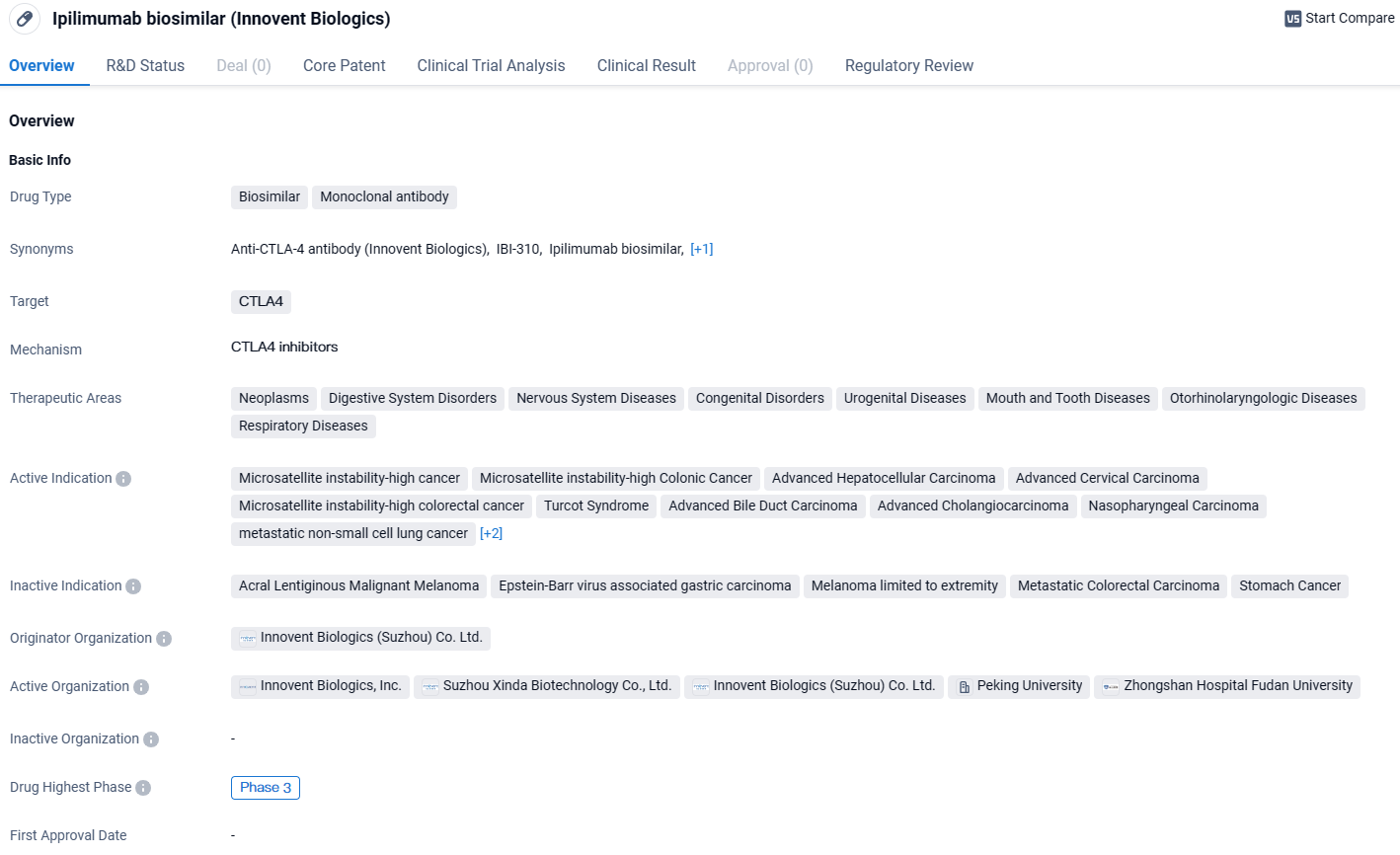
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In China, Neoshot represents the inaugural Phase 3 clinical investigation aimed at scrutinizing neoadjuvant immunotherapy for patients with MSI-H/dMMR colon cancer. The focus of this research is to ascertain the safety profile and therapeutic effectiveness of the combined use of IBI310 and sintilimab for neoadjuvant applications, which will be juxtaposed with the conventional adjuvant chemotherapeutic approach subsequent to complete surgical removal of MSI-H/dMMR colon cancer. The pivotal endpoints of the trial include the rate of pathologic complete response and the duration of event-free survival.
Earlier, during a Phase 1b randomized, controlled, and multicenter trial aimed at neoadjuvant treatment for individuals with surgically removable MSI-H/dMMR colon cancer, the group receiving both IBI310 and sintilimab displayed a notably greater pCR rate in comparison to patients treated solely with sintilimab. Importantly, none of the participants receiving the combination therapy were rendered inoperable owing to adverse effects, nor were there indications of heightened safety concerns. The outcomes of this Phase 1b study are expected to be disseminated at a forthcoming medical symposium or detailed in a peer-reviewed scientific publication.
Dr. Zhou Hui, a high-ranking executive at Innovent, has communicated the pressing necessity for novel neoadjuvant therapies for MSI-H/dMMR colon cancer that can be surgically removed within the Chinese context. Dr. Hui expressed contentment regarding the progress of the Phase 3 Neoshot trial, particularly with the initiation of treatment in the first patient. There is an anticipation for the upcoming conclusions of the trial, which are hoped to unveil greatly improved treatment avenues for MSI-H/dMMR colon cancer sufferers in China.
Developed in-house by Innovent, IBI310 is a highly specific, fully human monoclonal antibody. Its mode of action involves binding to cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a critical step in disengaging the CTLA-4 mediated inhibitory effect on T cells, which in turn shores up T cell activation and expansion. Such a mechanism bolsters the immune-mediated onslaught against tumors, thereby delivering antineoplastic benefits.
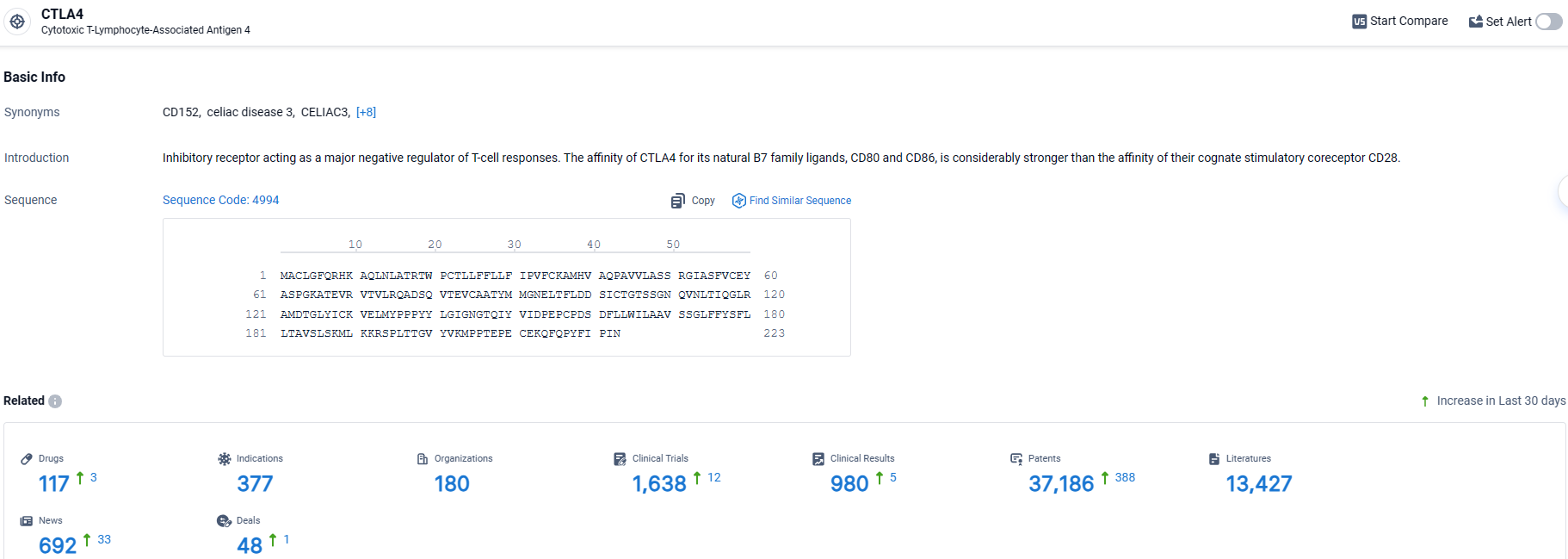
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 28 2024, there are 117 investigational drugs for the CTLA-4 target, including 377 indications, 180 R&D institutions involved, with related clinical trials reaching 1638, and as many as 37186 patents.
IBI310 has the potential to treat a wide range of therapeutic areas. The drug has reached Phase 3 of clinical trials globally and in China, indicating its advanced stage of development. Additionally, its breakthrough therapy designation further emphasizes its potential to address critical medical needs.