Inspirna partners with German Merck KGaA to accelerate global development of RGX-202, also called Ompenaclid
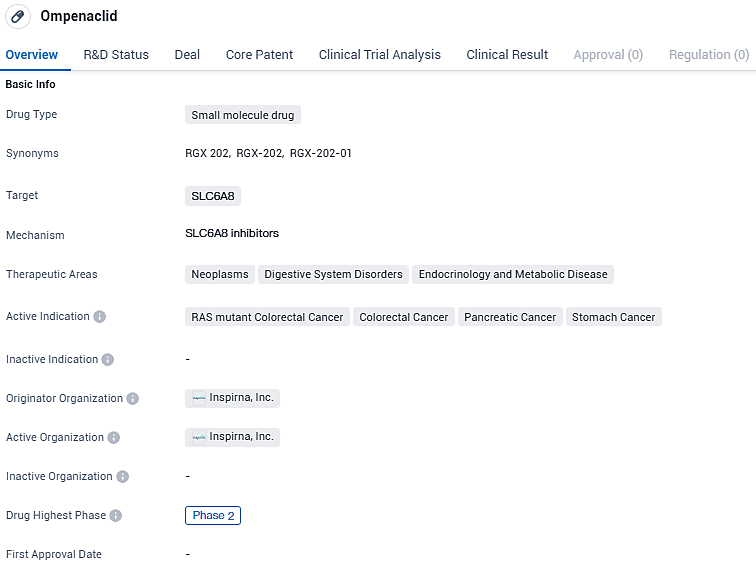
Inspirna, Inc., an enterprise operating in the clinical phase and specializing in the creation of pioneering oncology treatments, has disclosed a formal licensure pact with Merck KGaA, headquartered in Darmstadt, Germany. This agreement concerns the compound ompenaclid (RGX-202), which represents a novel category of orally administered agents aimed at inhibiting the creatine transport pathway SLC6A8. Ompenaclid is presently being tested for its efficacy in treating advanced or metastatic colorectal cancer in patients with RAS mutations, positioned as a second-line therapy. Additionally, the terms of the agreement encompass rights to subsequent medicinal compounds that also target SLC6a8.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
We're thrilled to announce our collaboration with Merck KGaA, Darmstadt, Germany, a renowned authority in the field of cancer therapy who possesses specialized knowledge and a worldwide reputation in the treatment of colorectal cancer, in our mission to deliver innovative treatments to a broader demographic of patients," stated Dr. Usman Azam, M.D., President and CEO of Inspirna.
"Our recent findings confirm our hypothesis that ompenaclid could represent a groundbreaking treatment for advanced stages of colorectal cancer, highlighting the effectiveness of our unique RNA-DRIVEr? technology for identifying novel targets. Our team is eager to enhance our partnership with Merck KGaA, Darmstadt, Germany, as we progress through a Phase 2 randomized clinical study of ompenaclid," expanded Dr. Azam.
"In the last ten years, advancements in addressing RAS-mutated colorectal cancer, which constitutes nearly half of the cases that require second-line treatment, have been minimal,” remarked Victoria Zazulina, M.D., Chief of the Development Unit within the Oncology sector at Merck KGaA, Darmstadt, Germany. "Leveraging our strength in colorectal cancer therapies, and considering the promising preliminary results obtained with ompenaclid, our alliance with Inspirna is poised to propel the development of what could be a pioneering therapy, potentially enhancing patient care."
As per the licensing deal, Merck KGaA, Darmstadt, Germany will be granted an exclusive license to distribute ompenaclid outside the United States, with the opportunity to join hands in its development and promotion within the US market. Additionally, both entities have agreed to co-operate on the subsequent generation of compounds that target SLC6A8, with Inspirna maintaining rights to co-develop and co-market within the US. Initially, Inspirna will be compensated with a payment of $45 million.
Upon reaching predefined developmental, regulatory, and sales benchmarks with ompenaclid, Inspirna could receive additional payments that increase progressively, accompanied by royalties on international net sales starting in the low teens. Moreover, Inspirna stands to gain payments upon meeting specific milestones in development, regulatory processes, and sales for each SLC6A8-targeted successor compound, plus royalties on international net sales that could reach into double figures.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
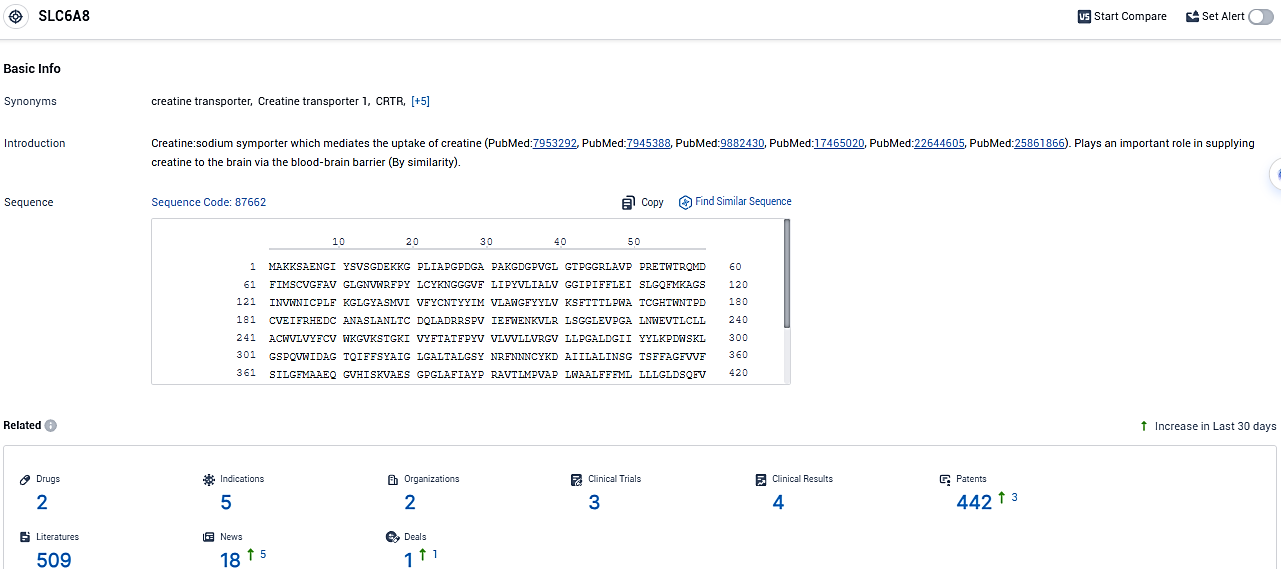
According to the data provided by the Synapse Database, As of January 9, 2024, there are 2 investigational drugs for the SLC6A8 target, including 5 indications, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 442 patents.
Ompenaclid targets SLC6A8 and is being investigated for its potential applications in neoplasms, digestive system disorders, and endocrinology and metabolic diseases. The drug is currently in Phase 2 of clinical development and is being studied for its efficacy in treating RAS mutant colorectal cancer, colorectal cancer, pancreatic cancer, and stomach cancer. Further research and clinical trials are needed to determine the drug's safety and effectiveness before it can be approved for use in patients.