Intellia Launches Phase 3 MAGNITUDE Trial for CRISPR Therapy NTLA-2001 in Cardiac Amyloidosis
Intellia Therapeutics, Inc., a prominent enterprise engaged in the advanced clinical development of gene modification solutions, has reported the commencement of treatment for the inaugural participant within their international, crucial Phase 3 trial named MAGNITUDE, which evaluates the efficacy of their therapeutic candidate NTLA-2001. This endeavor is centered on transforming medical treatments through the application of CRISPR technology.
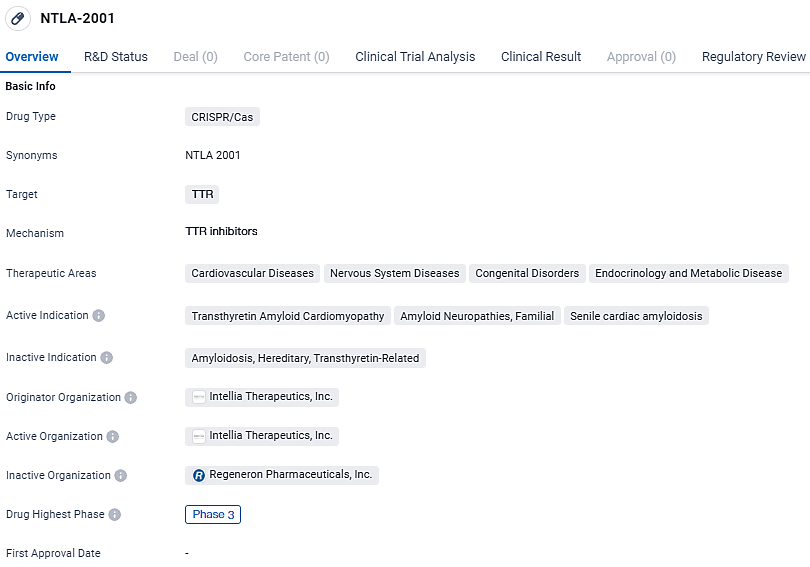
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
NTLA-2001 stands as an experimental gene-editing therapeutic based on in vivo CRISPR technology, tailored to serve as a one-off intervention aimed at disabling the TTR gene, hence obstructing the synthesis of the TTR protein in addressing transthyretin amyloidosis. The clinical study, titled MAGNITUDE, is currently assessing the effectiveness and safety profile of NTLA-2001 in subjects diagnosed with ATTR amyloidosis complicated by cardiomyopathy.
Intellia Therapeutics' top executive, John Leonard, M.D., highlighted the significance of the initiation of patient dosing within the MAGNITUDE study for NTLA-2001, marking a progressive step toward the potential development of a singular gene-editing therapy for those affected by ATTR amyloidosis.
Dr. Leonard expressed anticipation over the forthcoming insights into NTLA-2001's performance and its safety in treating cardiomyopathy within the crucial Phase 3 clinical trial. He held that favorable results could lay the foundation for subsequent international regulatory submissions, strongly aligned with the company's mission to introduce an innovative treatment to the ATTR amyloidosis patient population.
Julian Gillmore, M.D., Ph.D., an esteemed Medicine Professor at the National Amyloidosis Centre at UCL, affiliated with the Royal Free Hospital in the U.K., and the appointed coordinator for the Phase 3 trial on a national level, conveyed his team’s excitement to be at the forefront in administering NTLA-2001 to a patient. He underscored the potential this in vivo CRISPR-based therapy has to revolutionize care standards for individuals battling this severe illness.
As a ground-breaking therapeutic candidate and the first of its kind, NTLA-2001 targets the deactivation of the gene responsible for producing transthyretin. Promising early stage clinical results from Phase 1 trials suggest that NTLA-2001 has consistently achieved substantial and enduring suppression of transthyretin levels post-treatment.
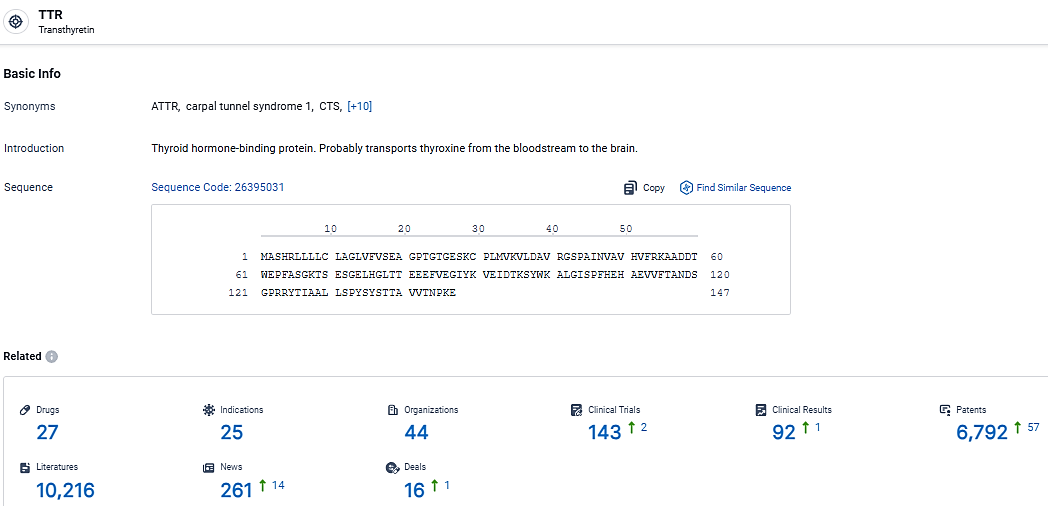
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 20 2024, there are 27 investigational drugs for the TTR target, including 25 indications, 44 R&D institutions involved, with related clinical trials reaching 143, and as many as 6792 patents.
NTLA-2001 represents a significant advancement in the field of biomedicine. Its utilization of CRISPR/Cas technology and focus on targeting TTR protein opens up possibilities for treating various diseases within the cardiovascular, nervous system, congenital, and endocrinology and metabolic disease areas. The drug's progression to Phase 3 trials indicates promising results in earlier stages, and its orphan drug status highlights its potential to address unmet medical needs in rare diseases.