Invivyd Initiates Phase 1 Trial of New COVID-19 Monoclonal Antibody VYD2311, Building on PEMGARDA™ Success
Invivyd, Inc. (Nasdaq: IVVD), a company specializing in biopharmaceuticals and targeting protection from severe viral infections, has started administering doses to participants in a Phase 1 clinical trial. This trial, which involves healthy volunteers, is focused on VYD2311, a highly promising monoclonal antibody (mAb) candidate designed to combat COVID-19. VYD2311 has shown strong neutralizing effects in vitro against the COVID-19 variants that have emerged since Omicron.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Phase 1 randomized, blind, placebo-controlled study is designed to evaluate escalating doses, alongside the safety, tolerability, pharmacokinetics, and immunogenicity of VYD2311 in healthy subjects (NCT06523153). Conducted in Australia, this Phase 1 trial will explore various dose levels of VYD2311, employing different administration routes, including intramuscular (IM) delivery, which aims to be more convenient for both the system and patients compared to intravenous approaches. Consistent with the company's broader strategy, VYD2311 was developed via affinity maturation to target newer variants of SARS-CoV-2. The anticipation is that preliminary data from this Phase 1 trial will be available in Q4 2024, with further clinical data from the VYD2311 program anticipated in 2025.
"We are excited about the potential for VYD2311 to build upon the successes of PEMGARDA™ (pemivibart), which received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for pre-exposure prophylaxis (PrEP) for COVID-19 in certain immunocompromised individuals,” said Marc Elia, Chairman of the Board. "COVID-19 and its long-term impacts continue to pose a global threat. At Invivyd, we are dedicated to developing new therapeutics that keep pace with the virus's evolution, aiming to protect those who need more comprehensive protection than that provided by booster vaccinations."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
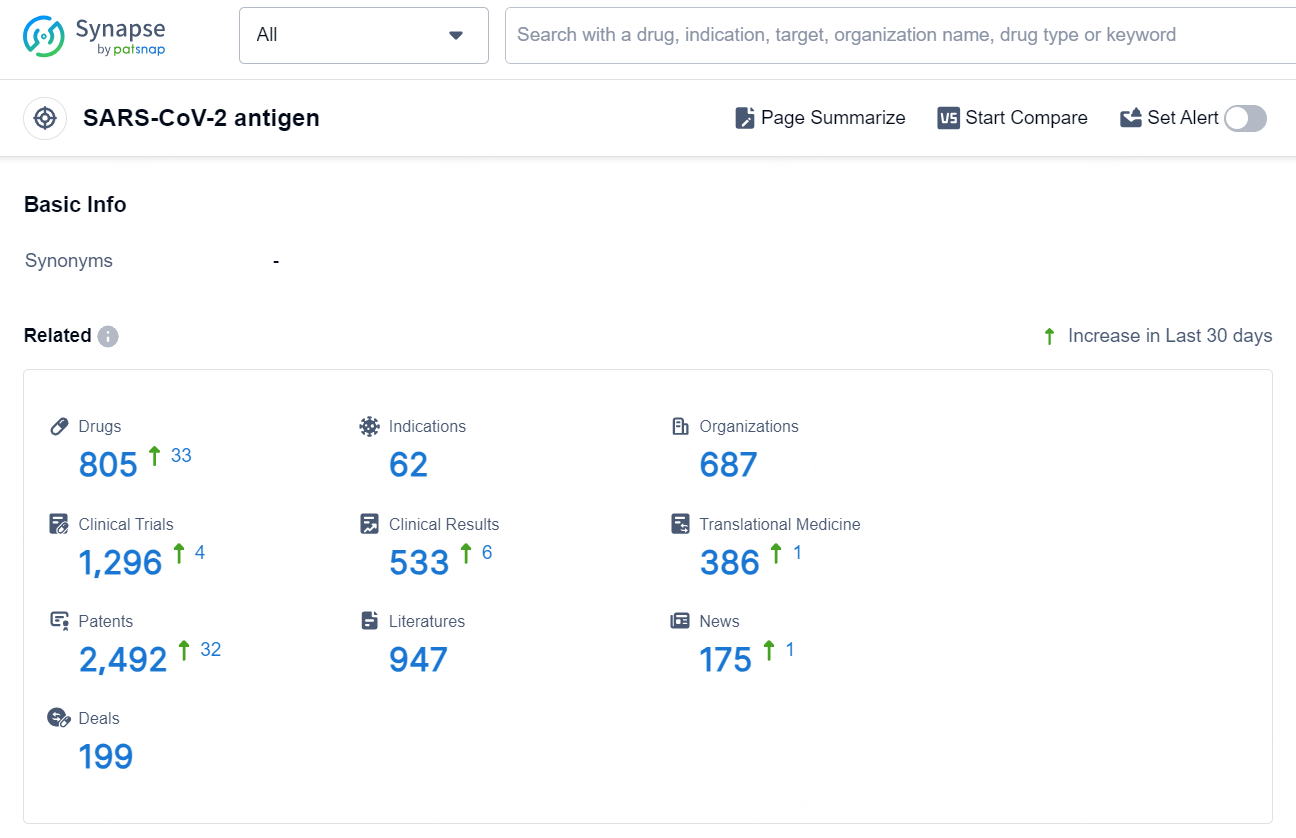
According to the data provided by the Synapse Database, As of September 9, 2024, there are 805 investigational drugs for the SARS-CoV-2 antigen target, including 62 indications, 687 R&D institutions involved, with related clinical trials reaching 1296, and as many as 2492 patents.
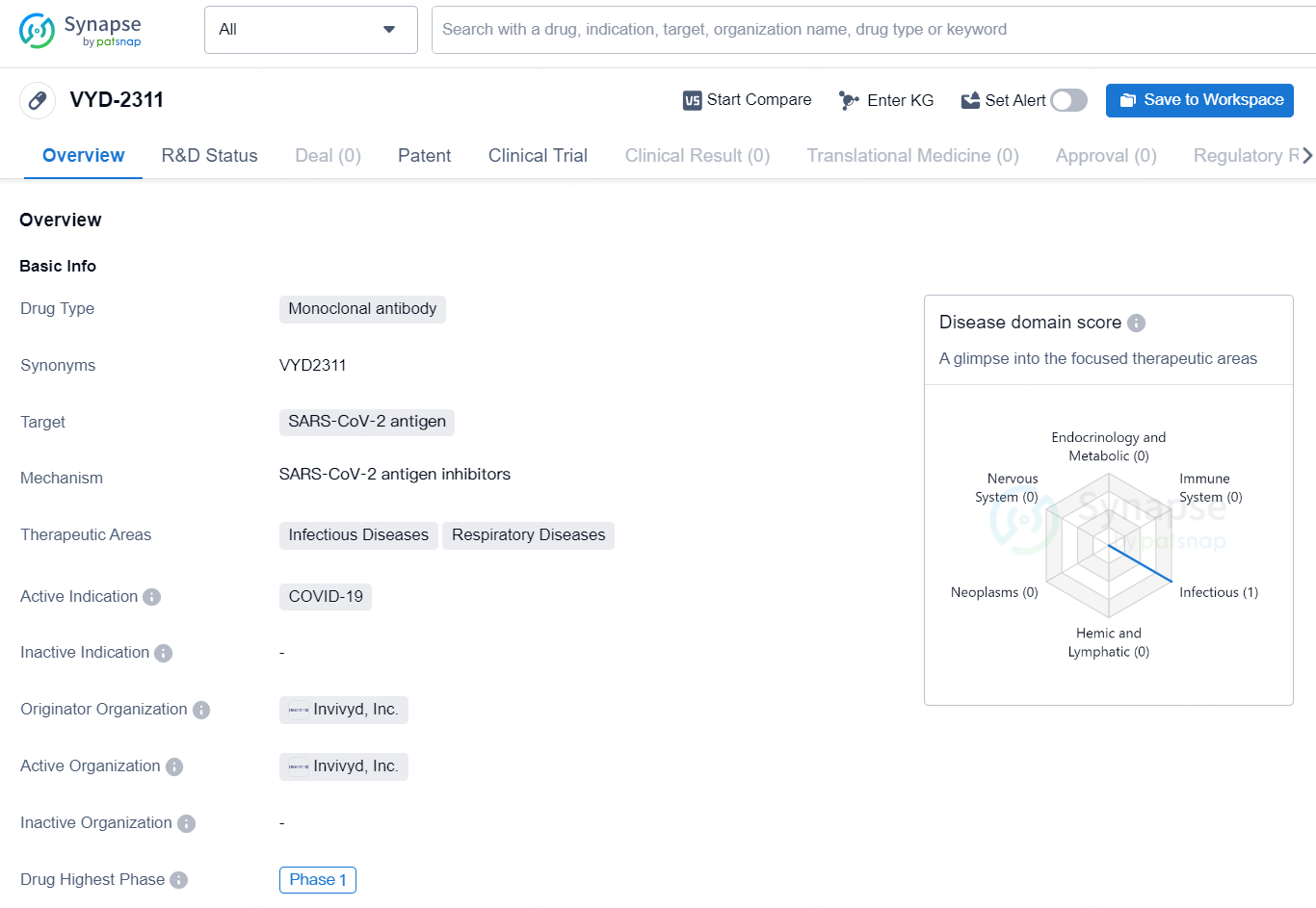
The drug VYD-2311 is a monoclonal antibody that targets the SARS-CoV-2 antigen, making it a potential candidate for treating COVID-19, particularly in the areas of infectious diseases and respiratory diseases. The drug is being developed by Invivyd, Inc. and has reached the highest phase of Phase 1 globally. Monoclonal antibodies are a type of targeted therapy used to treat certain types of cancer and autoimmune diseases. They work by targeting specific proteins on the surface of cells. In the case of VYD-2311, it targets the antigen of the SARS-CoV-2 virus, which is responsible for causing COVID-19. This indicates that the drug is specifically designed to combat the viral infection and may offer potential therapeutic benefits.