Ionis reveals favorable topline outcomes from Phase 1/2a study of ION582 for Angelman syndrome
Ionis Pharmaceuticals, Inc. reported favorable primary results from the HALOS Phase 1/2a open-label trial of ION582 for Angelman syndrome. The study demonstrated that ION582 was both safe and well tolerated, showing promising and consistent advantages for individuals with Angelman syndrome. Significant improvements were particularly noted in critical areas such as cognitive abilities, communication skills, and motor functions.
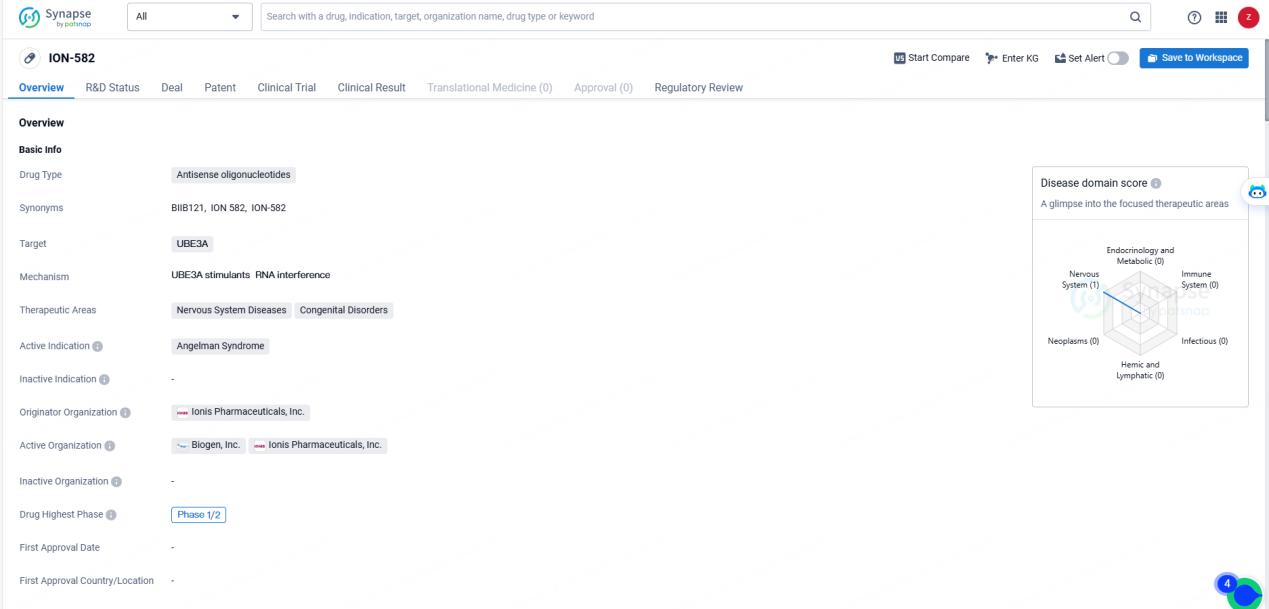
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Ionis declared that it will independently progress with ION582 as a part of its premier portfolio of potentially groundbreaking treatments for severe neurological disorders. The company intends to discuss the Phase 1/2a results of ION582 with regulatory bodies to agree on the pivotal program’s design. Biogen has decided against exercising its option to license ION582.
"There are currently no approved therapies for Angelman syndrome, a condition that leads to developmental delays, cognitive impairments, and significant communication challenges, with most affected individuals unable to speak or live independently,” commented Lynne Bird, M.D., professor of clinical pediatrics at UC San Diego and an investigator in the HALOS study.
“We are heartened by the consistent positive outcomes observed in multiple measures within the HALOS study, as these functional enhancements could profoundly impact the lives of those with Angelman syndrome and their caregivers. Furthermore, the favorable safety and tolerability profile noted in the study is particularly crucial for treating children. We eagerly anticipate further assessment of ION582 in a pivotal program,” added Lynne Bird.
Angelman syndrome is attributed to a loss of function in the maternal UBE3A gene. ION582 aims to activate the paternal UBE3A allele to boost the production of the UBE3A protein in the brain. This syndrome affects roughly one in every 12,000 to 20,000 people worldwide, presenting as severe developmental delays in motor, language, and cognitive skills, along with seizures and ataxia. It is a critical neurodevelopmental disorder that emerges early in life, rendering individuals completely dependent on caregivers.
“We are encouraged by the data from the HALOS study and delighted to include this promising treatment in our expanding independent pipeline of neurological medications,” stated Brett Monia, Ph.D., CEO of Ionis. "We look forward to sharing detailed data at the upcoming Angelman Syndrome Foundation meeting and to advancing ION582 into a pivotal study. Individuals with Angelman syndrome face substantial neuro-developmental challenges and currently have no approved treatments. We are committed to working closely with the community, researchers, and regulatory authorities to advance this promising investigational medication."
ION582 exhibited consistent effects across various objective and subjective measures for evaluating functioning in individuals with Angelman syndrome. These measures include the Bayley-4, an objective physician assessment of clinical functioning, the Angelman Syndrome Clinical Global Improvement Change scale for evaluating clinicians’ impressions, as well as the Vineland-3 and Observer-Reported Communication Ability measures, which are tools for parent-reported assessments.
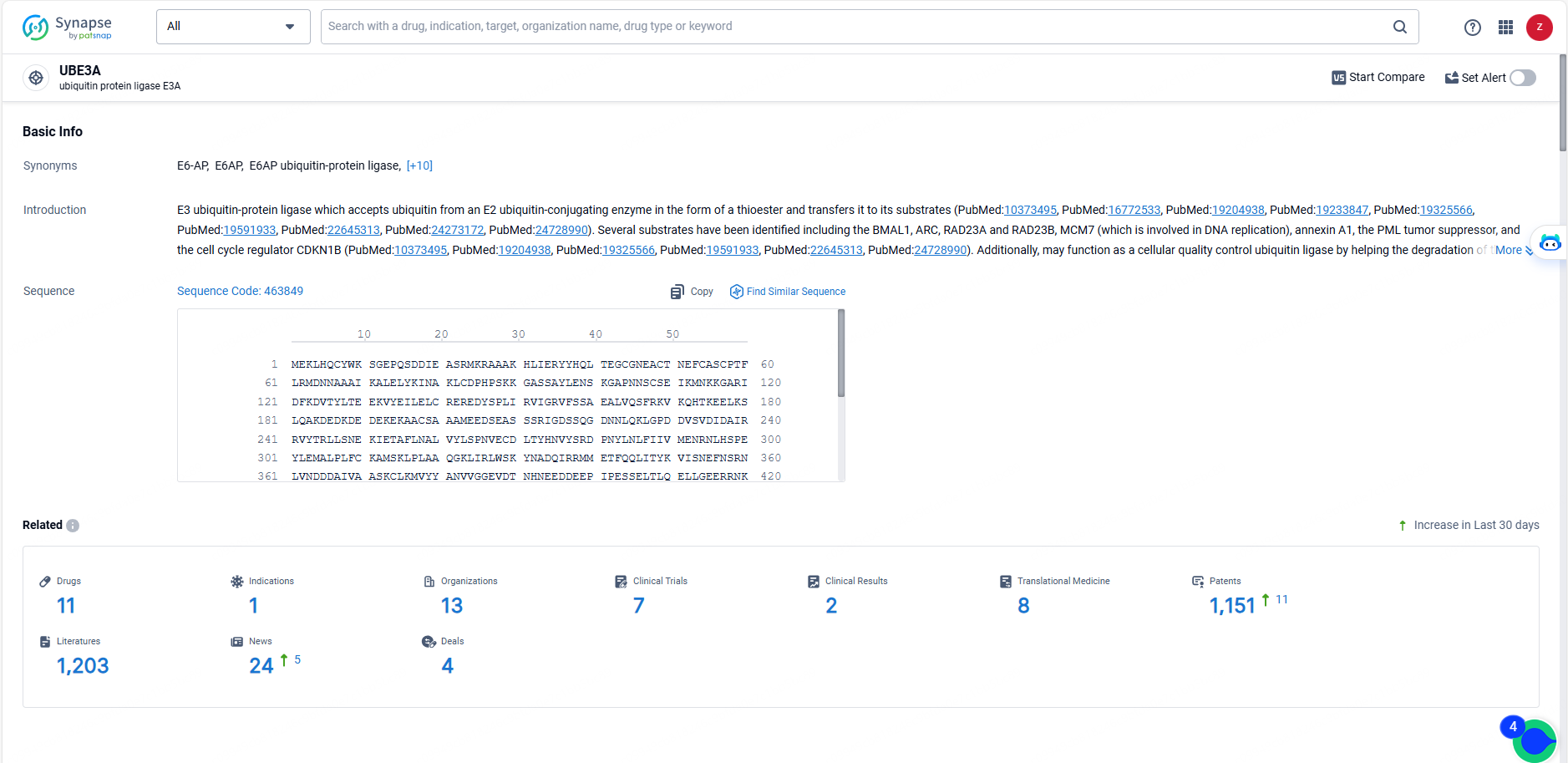
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 11 investigational drugs for the UBE3A targets, including 1 indications, 13 R&D institutions involved, with related clinical trials reaching 7, and as many as 1151 patents.
ION-582 holds promise as a potential treatment for Angelman Syndrome, and its progress through clinical development and regulatory designations underscores the importance of addressing unmet medical needs in rare pediatric diseases. As the drug continues to advance, it may offer hope for patients and families affected by this challenging condition.