Is Belzutifan approved by the FDA?
Yes, Belzutifan is FDA approved. The U.S. Food and Drug Administration (FDA) approved Belzutifan on August 13, 2021, for the treatment of adult patients with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET) that do not require immediate surgery.
Uses and Benefits
Belzutifan is specifically designed to treat tumors associated with VHL disease in adults. VHL is a genetic disorder characterized by the formation of tumors and cysts in different parts of the body, including the kidneys, pancreas, and central nervous system. Belzutifan works by inhibiting hypoxia-inducible factor-2 alpha (HIF-2α), which is involved in the growth of these tumors.
Administration and Dosage
Belzutifan is administered as follows:
- Dosage Form: Oral tablet (40 mg)
- Usual Adult Dose for Von Hippel-Lindau Syndrome: 120 mg orally once daily until disease progression or unacceptable toxicity.
The medication should be taken at the same time each day, with or without food. The tablets should be swallowed whole and not crushed, chewed, or broken. If a dose is missed, it should be taken on the same day as remembered but not taken twice in one day.
Side Effects
Belzutifan can cause several side effects, some of which may be serious. Common side effects include:
- Headache
- Dizziness
- Tiredness
- Nausea
- Increased blood sugar levels
- Abnormal kidney function tests
Serious side effects that require immediate medical attention include:
- Anemia (low red blood cells): Symptoms include pale skin, tiredness, feeling light-headed or short of breath, cold hands and feet.
- Low oxygen levels: Symptoms include shortness of breath, chest pain, fast heartbeats.
Patients should contact their doctor immediately if they experience any severe side effects. Your cancer treatments may be delayed or permanently discontinued if you have certain side effects.
Precautions
Before starting treatment with Belzutifan, patients should consider the following precautions:
- Medical Conditions: Inform your doctor if you have ever had low red blood cells.
- Pregnancy and Birth Control: Belzutifan may harm an unborn baby. Both men and women using Belzutifan should use effective non-hormonal birth control during treatment and for at least one week after the last dose. Hormonal birth control methods may be less effective with Belzutifan.
- Breastfeeding: Do not breastfeed while using this medicine and for at least one week after your last dose.
Conclusion
Belzutifan provides a significant treatment option for adults with von Hippel-Lindau disease who need therapy for certain associated tumors. It is important to follow the prescribed administration guidelines, take necessary precautions, and consult with healthcare providers for personalized medical advice and treatment plans.
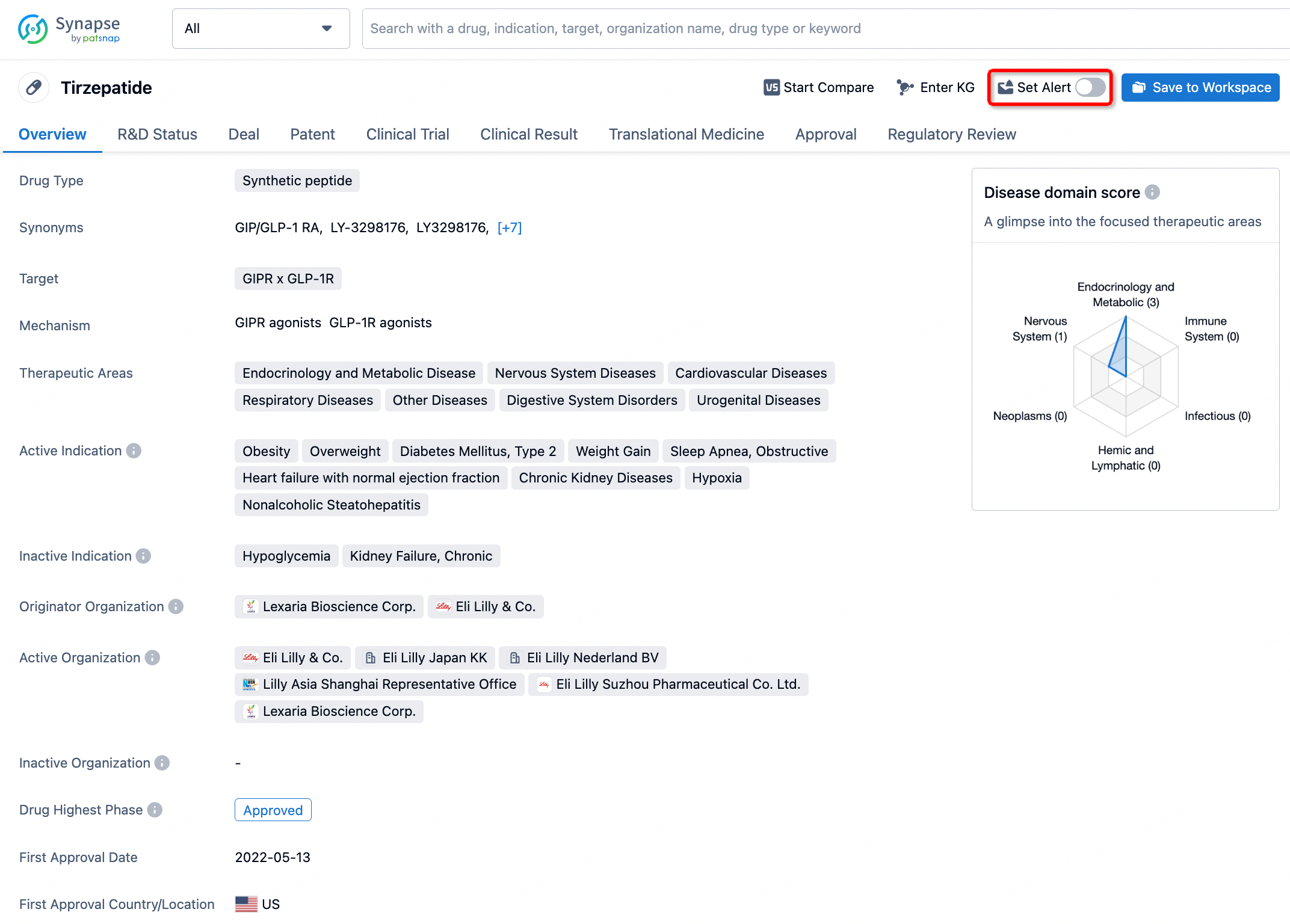
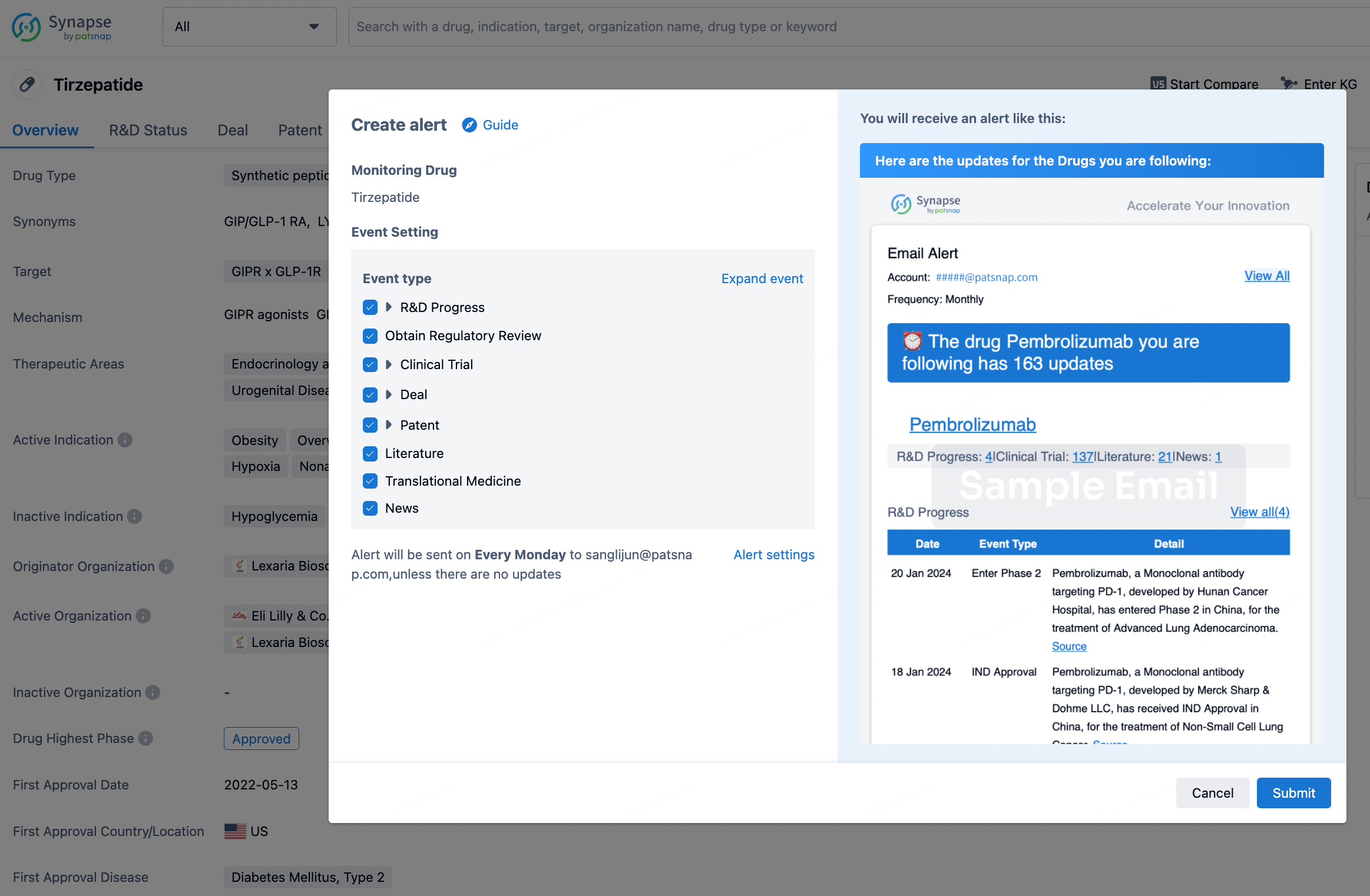
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!