Is Cedazuridine/Decitabine approved by the FDA?
Yes, the combination of decitabine and cedazuridine, marketed under the brand name Inqovi, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Inqovi on July 7, 2020, for the treatment of certain types of myelodysplastic syndromes (MDS).
What is Cedazuridine and Decitabine?
Cedazuridine and decitabine is an oral combination medication used to treat myelodysplastic syndromes, which are a group of disorders caused by poorly formed or dysfunctional blood cells. This combination is classified under antineoplastic combinations, and it is specifically indicated for certain French-American-British subtypes of MDS, including refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, and chronic myelomonocytic leukemia (CMML). It is used in intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.
Dosage and Administration
The usual adult dose for myelodysplastic syndrome is one tablet (containing 100 mg of cedazuridine and 35 mg of decitabine) taken orally once daily on Days 1 through 5 of each 28-day cycle. Treatment should continue for a minimum of four cycles, and a complete or partial response may take longer than four cycles. The medication should be taken on an empty stomach, at least 2 hours before or 2 hours after a meal.
Important Warnings and Precautions
Serious Side Effects:
- Easy bruising, unusual bleeding, purple or red spots under the skin
- Low red blood cells (anemia), which can cause pale skin, unusual tiredness, feeling light-headed or short of breath, cold hands and feet
- Low white blood cell counts, which can lead to fever, mouth sores, skin sores, sore throat, cough, trouble breathing
- Signs of a lung infection such as fever, chills, cough with mucus, chest pain, and shortness of breath
Common Side Effects:
- Fever, low blood cell counts
- Bruising or bleeding
- Abnormal liver function tests
- Headache, dizziness, feeling tired
- Swelling in arms or legs
- Muscle or joint pain
- Painful mouth sores
- Shortness of breath, lung infection
- Nausea, loss of appetite
- Diarrhea, constipation
- Rash
- Cold symptoms such as stuffy nose, sneezing, cough, sore throat
Cedazuridine and decitabine affect the immune system, making infections more likely. It's important to monitor for symptoms such as fever, chills, cough, mouth sores, or unusual bleeding or bruising.
Special Considerations
Pregnancy and Breastfeeding:
- Cedazuridine and decitabine can harm an unborn baby or cause birth defects. Women should not use this medication if pregnant and should use effective birth control during treatment and for at least six months after the last dose. Men should also use effective birth control if their partner can become pregnant and continue for at least three months after the last dose.
- This medication may affect fertility in men.
- Breastfeeding should be avoided during treatment and for at least two weeks after the last dose.
Contraindications:
- Not approved for use in individuals younger than 18 years old.
- Patients with kidney or liver disease should inform their doctor before starting treatment.
Conclusion
Decitabine and cedazuridine (Inqovi) received FDA approval on July 7, 2020, for the treatment of myelodysplastic syndromes. It is an important treatment option for managing these disorders and is taken as an oral tablet in specified cycles. Patients should be aware of the potential serious and common side effects and should follow their healthcare provider's instructions carefully to manage their condition effectively.
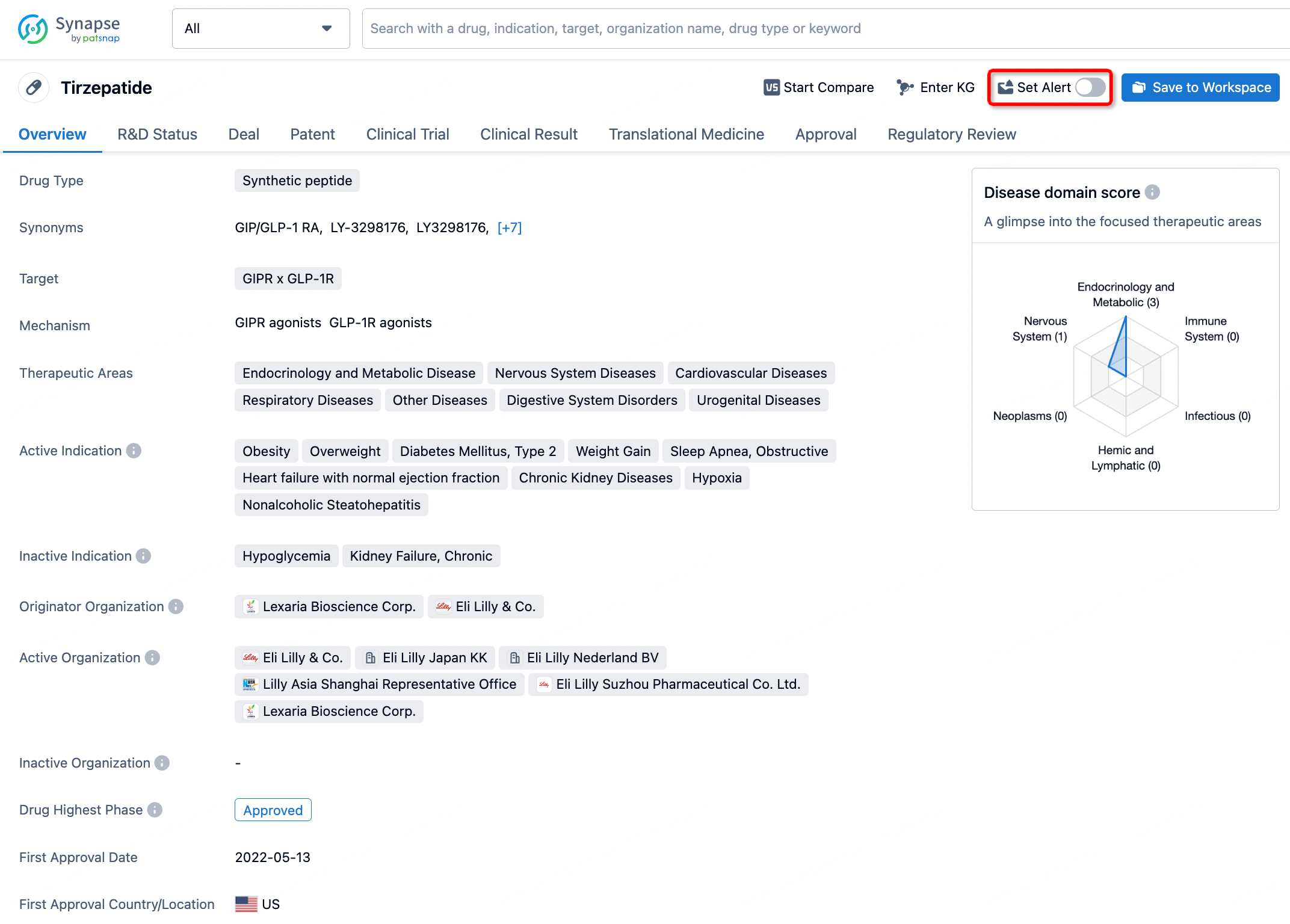
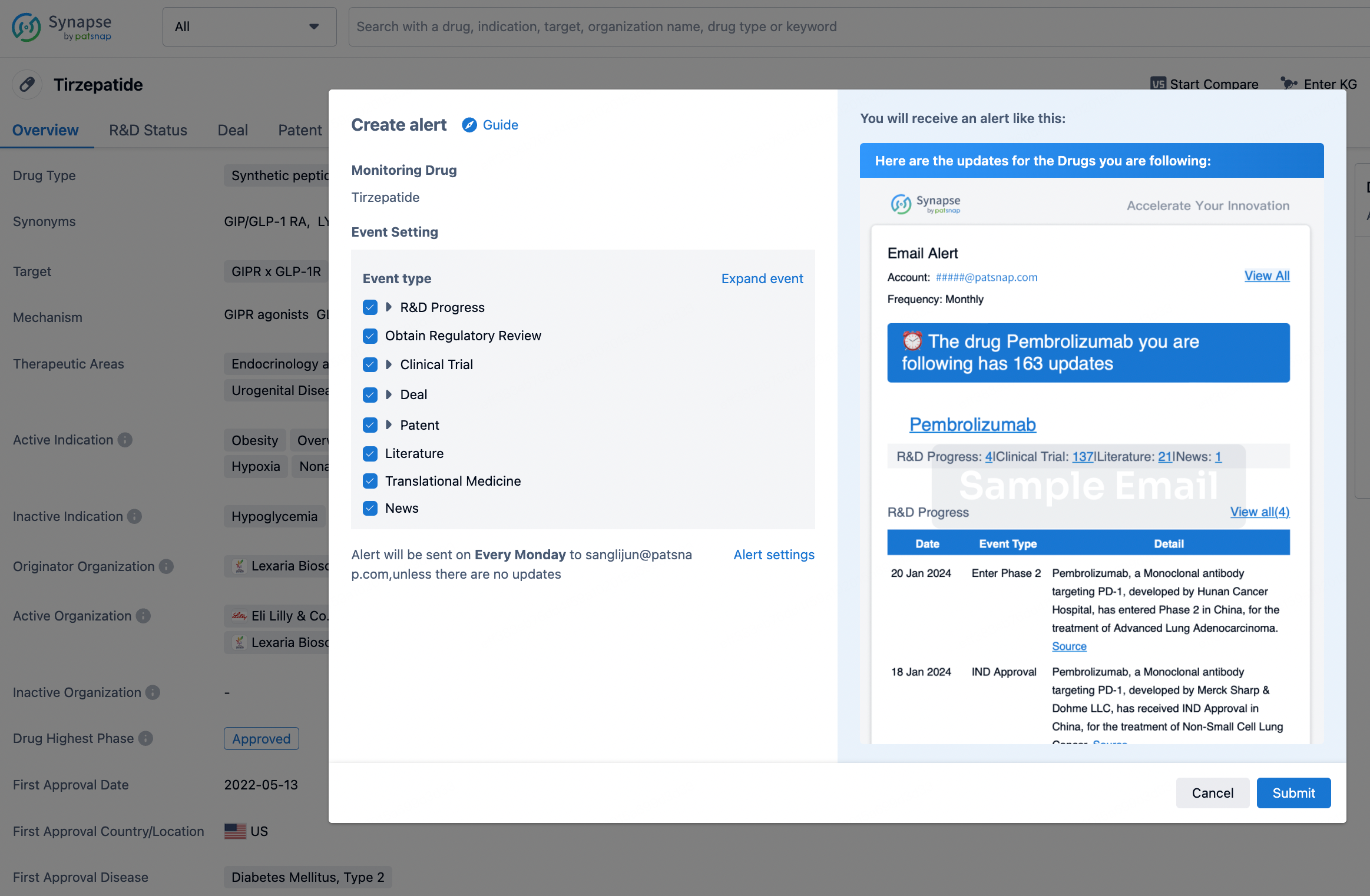
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!