Is Lemborexant approved by the FDA?
Yes, lemborexant, marketed under the brand name Dayvigo, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Dayvigo on December 20, 2019, for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance in adults.
What is Lemborexant?
Lemborexant is a medication used to treat insomnia, which is the difficulty of falling or staying asleep. It belongs to the drug class of miscellaneous anxiolytics, sedatives, and hypnotics. It works by targeting and blocking the orexin receptors, which play a role in regulating the sleep-wake cycle.
Dosage and Administration
Lemborexant is available in oral tablet form with dosages of 5 mg and 10 mg. The usual adult dose for insomnia is 5 mg taken once daily at bedtime. If needed, the dose can be increased to a maximum of 10 mg. It is important to take lemborexant only once per night, right before going to bed, and ensure that there are at least 7 hours available for sleep before becoming active again.
Side Effects
Lemborexant may cause side effects, some of which can be serious. Common side effects include:
- Drowsiness during the day
- Headache
- Fatigue
Serious side effects may include:
- Trouble moving or talking when first waking up
- Unusual thoughts or behavior
- Memory problems
- Worsening depression or thoughts about hurting oneself
Warnings and Precautions
- Do not take lemborexant if you have narcolepsy or severe liver disease.
- It can cause drowsiness the next day, affecting activities that require full alertness, such as driving.
- It should not be taken with alcohol or other CNS depressants.
- People with a history of depression, mental illness, or suicidal thoughts should use it cautiously.
Usage Considerations
- Lemborexant can be habit-forming, so it should be used exactly as prescribed to avoid dependency or misuse.
- Avoid taking it shortly after a meal, as this may delay its effect.
- If insomnia does not improve within 7 to 10 days, consult your doctor.
Conclusion
Lemborexant, known as Dayvigo, is FDA approved for the treatment of insomnia in adults, offering a new option for those struggling with sleep disorders. Its approval on December 20, 2019, marked an important development in sleep medicine, providing an effective treatment option with specific usage guidelines and potential side effects that should be closely monitored.
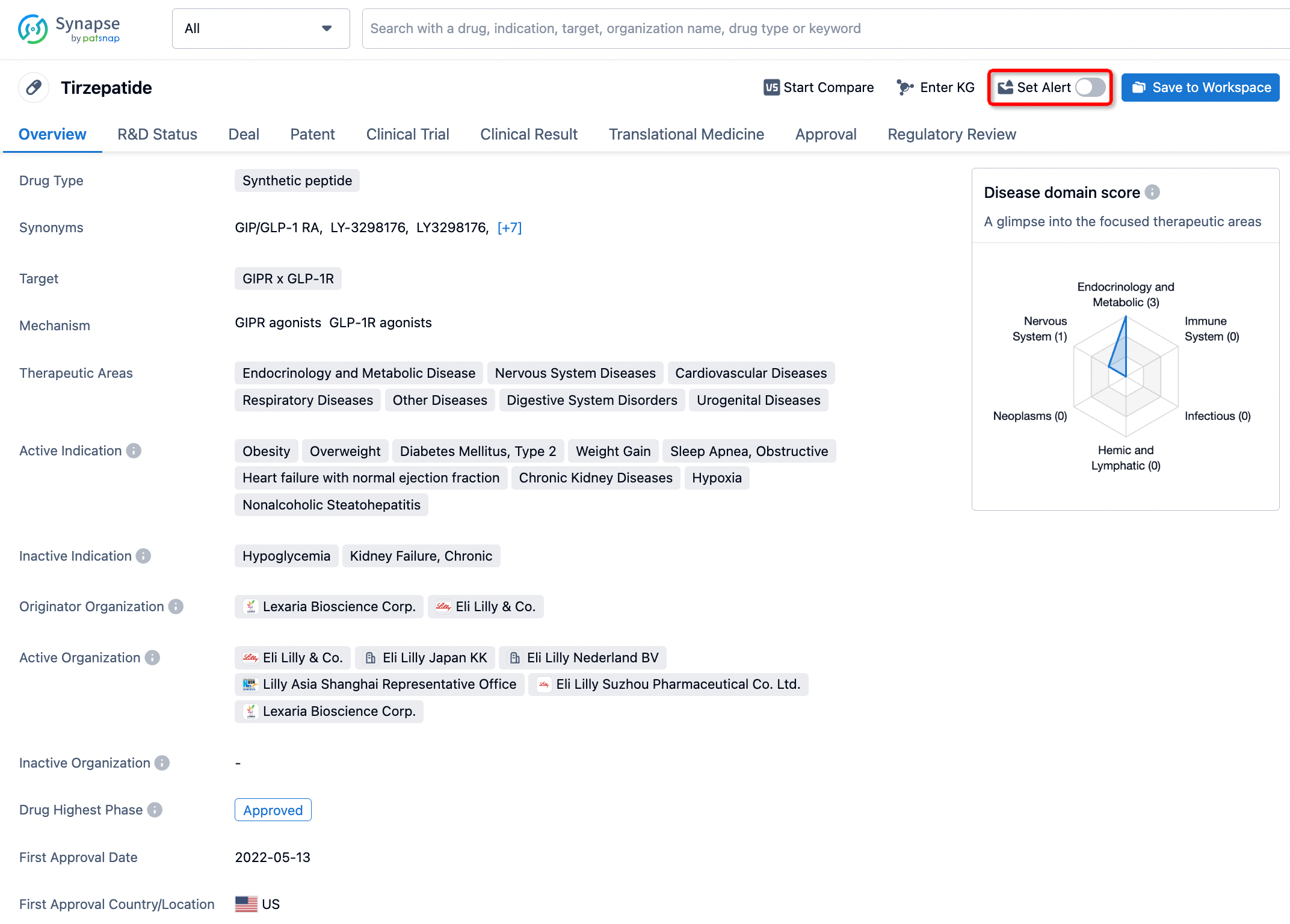
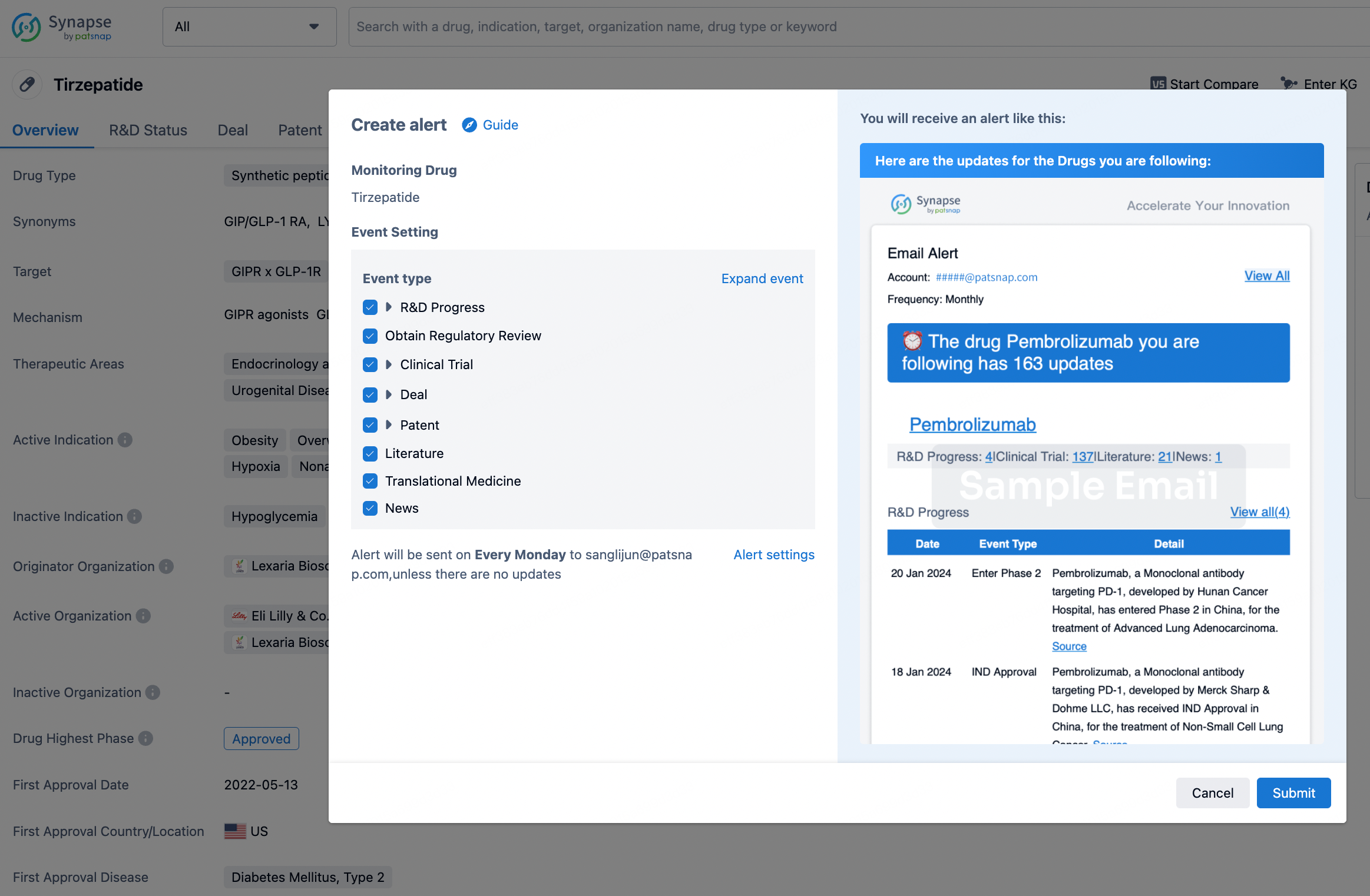
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!