Is Leniolisib approved by the FDA?
Yes, leniolisib, marketed under the brand name Joenja, is FDA approved. It received approval on March 24, 2023, for the treatment of activated phosphoinositide 3-kinase delta syndrome (APDS) in individuals aged 12 years and older.
Dosage and Administration
- Recommended Dosage: 70 mg taken orally twice daily, approximately 12 hours apart. It can be taken with or without food.
- If vomiting occurs more than one hour after taking leniolisib, do not take an additional dose. Simply take the next scheduled dose.
Side Effects
Common Side Effects
- Headache
- Dry or thickened skin, itching, rash
- Cold symptoms such as stuffy nose, sneezing, sore throat
Serious Side Effects
Seek immediate medical attention if you experience signs of an allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat. Serious side effects may include liver problems, indicated by symptoms such as:
- Loss of appetite
- Nausea
- Vomiting
- Upper right stomach pain
- Tiredness
- Itching
- Dark urine
- Clay-colored stools
- Jaundice (yellowing of the skin or eyes)
Warnings and Precautions
- Liver Disease: Inform your doctor if you have a history of liver disease.
- Pregnancy and Breastfeeding: A pregnancy test is required before starting treatment. Leniolisib can harm an unborn baby, so it is crucial to use effective birth control during treatment and for at least one week after the last dose. Do not breastfeed while using leniolisib and for at least one week after the last dose.
- Vaccines: Avoid receiving live vaccines during treatment, as the vaccine may not work as effectively.
Drug Interactions
Many drugs can interact with leniolisib, including prescription and over-the-counter medicines, vitamins, and herbal products. It is essential to inform your doctor about all the medications you are taking to avoid potential interactions.
Monitoring and Follow-Up
Regular monitoring of liver function and other blood parameters is recommended during treatment with leniolisib. Always follow your healthcare provider's instructions and report any side effects or concerns promptly.
Leniolisib provides a new treatment option for those suffering from APDS, contributing to improved management of this rare condition.
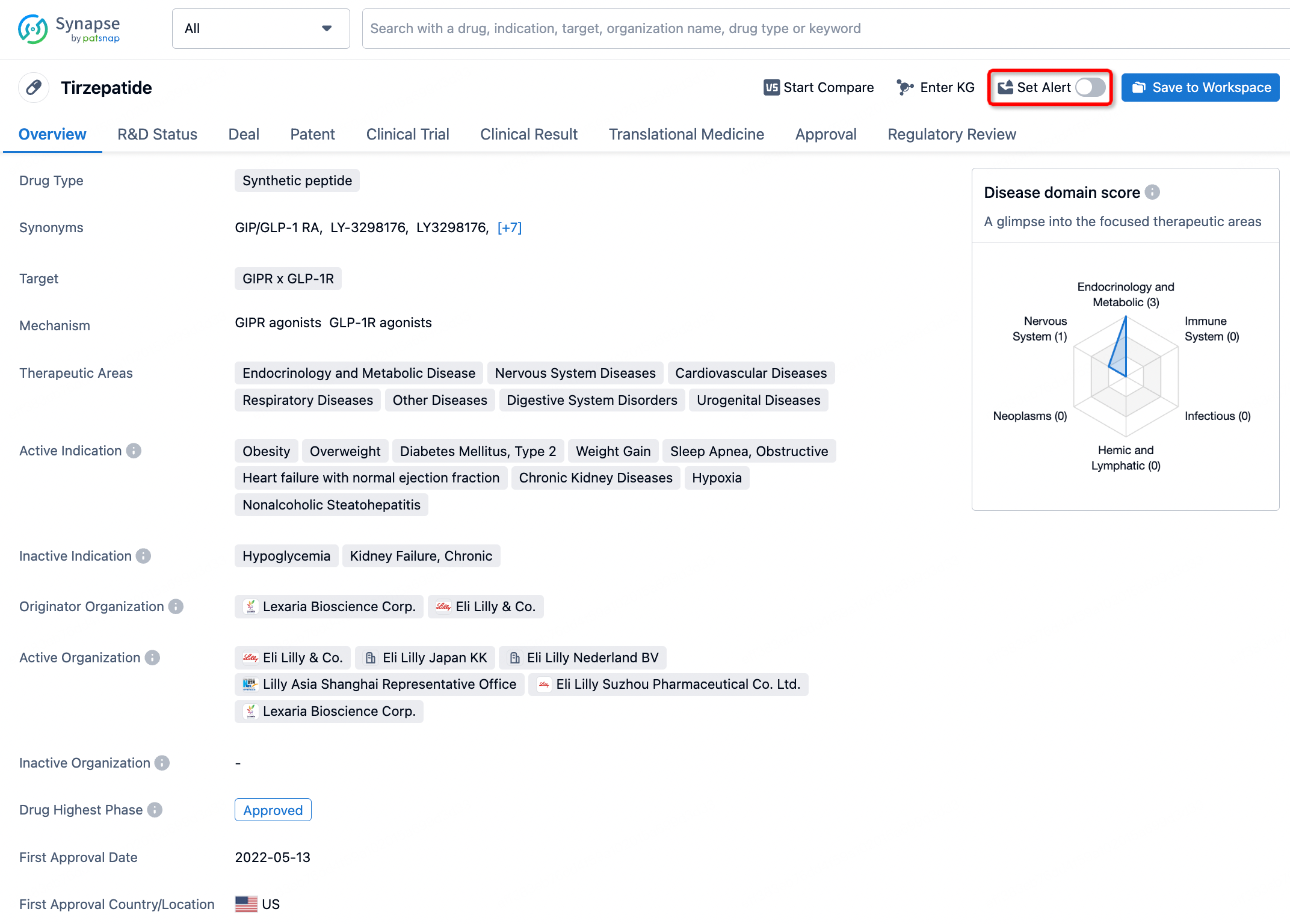
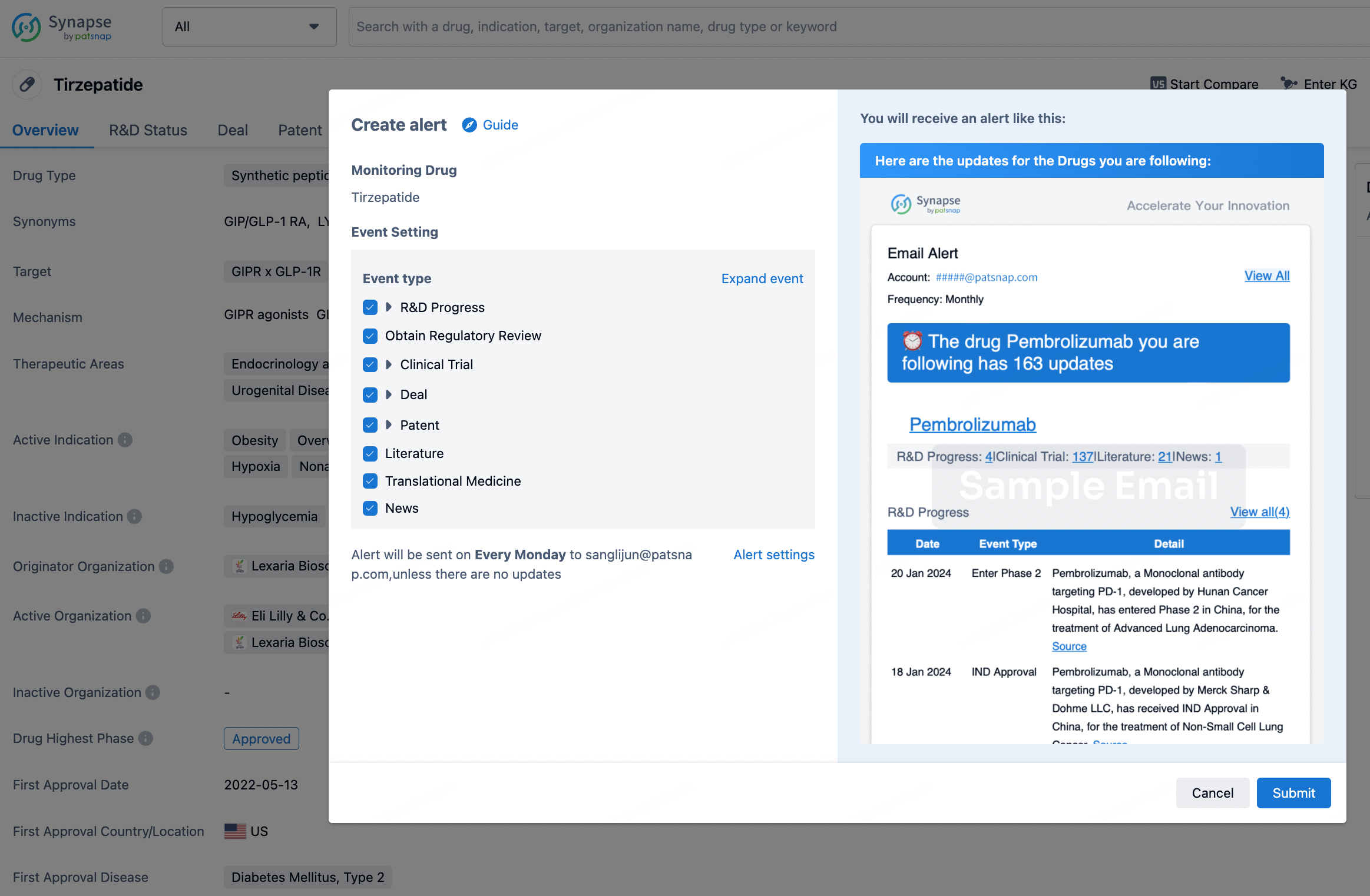
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!