Is Teplizumab approved by the FDA?
Teplizumab, marketed under the brand name Tzield, was approved by the FDA on November 17, 2022. This medication is used to delay the onset of stage 3 type 1 diabetes in individuals who are at least 8 years old and have stage 2 type 1 diabetes.
Usage: Teplizumab is administered as an intravenous infusion over approximately 30 minutes. The treatment involves daily infusions for 14 consecutive days. Prior to starting the therapy, your doctor will conduct tests to confirm the presence of stage 2 type 1 diabetes.
Side Effects: Patients should be aware of potential side effects, which can range from mild to severe. Common side effects include:
- Rash
- Headache
- Fever
- Mouth sores
- Skin sores
- Sore throat
- Cough
More serious side effects requiring immediate medical attention include:
- Signs of cytokine release syndrome (CRS) such as fever, chills, trouble breathing, confusion, severe vomiting or diarrhea, and irregular heartbeats
- Signs of infection including fever, chills, sore throat, body aches, unusual tiredness, and loss of appetite
- Liver problems such as loss of appetite, stomach pain (upper right side), itching, dark urine, clay-colored stools, and jaundice (yellowing of the skin or eyes)
Warnings:
- Inform your healthcare providers if you have a serious infection, an infection that keeps returning, or if you have recently received or are scheduled to receive a vaccine.
- Teplizumab may harm an unborn baby. Avoid use if you are pregnant and for at least 30 days before a planned pregnancy. Contact the Adverse Event reporting line if exposed to teplizumab during pregnancy.
- Do not breastfeed while using teplizumab. If considering breastfeeding, use a breast pump and discard any milk collected during treatment and for at least 20 days after the last dose.
Before Taking This Medicine: Before starting treatment with teplizumab, discuss with your doctor if you have:
- A serious infection or an infection that keeps returning or does not go away
- Recently received or are scheduled to receive a vaccine
Administration: Teplizumab is given as an infusion into a vein once daily for 14 days. It is important to follow all prescription label instructions and read all medication guides or instruction sheets. Your doctor may adjust, delay, or permanently discontinue your treatment based on side effects.
Dosing Information: The usual dose is based on body surface area and is administered by intravenous infusion once daily:
- Day 1: 65 mcg/m²
- Day 2: 125 mcg/m²
- Day 3: 250 mcg/m²
- Day 4: 500 mcg/m²
- Days 5 through 14: 1,030 mcg/m²
Precautions:
- Confirm stage 2 type 1 diabetes through appropriate tests before starting treatment.
- Conduct complete blood count and liver enzyme tests before initiating treatment.
- Ensure all age-appropriate vaccinations are completed before starting treatment.
- Premedication with a nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen, an antihistamine, and/or an antiemetic is recommended before the first 5 doses to prevent serious side effects.
What to Avoid: Avoid receiving inactivated, mRNA, and "live" vaccines during treatment with teplizumab, as the vaccine may not work as effectively.
Drug Interactions: Teplizumab may interact with other medications, including prescription and over-the-counter medicines, vitamins, and herbal products. Inform your doctor about all the medicines you use to avoid harmful interactions.
Conclusion: Teplizumab (Tzield) is FDA approved for delaying the onset of stage 3 type 1 diabetes in individuals 8 years and older with stage 2 type 1 diabetes. Patients should be aware of potential side effects and interactions with other medications. Regular monitoring and communication with healthcare providers are essential for safe and effective treatment.
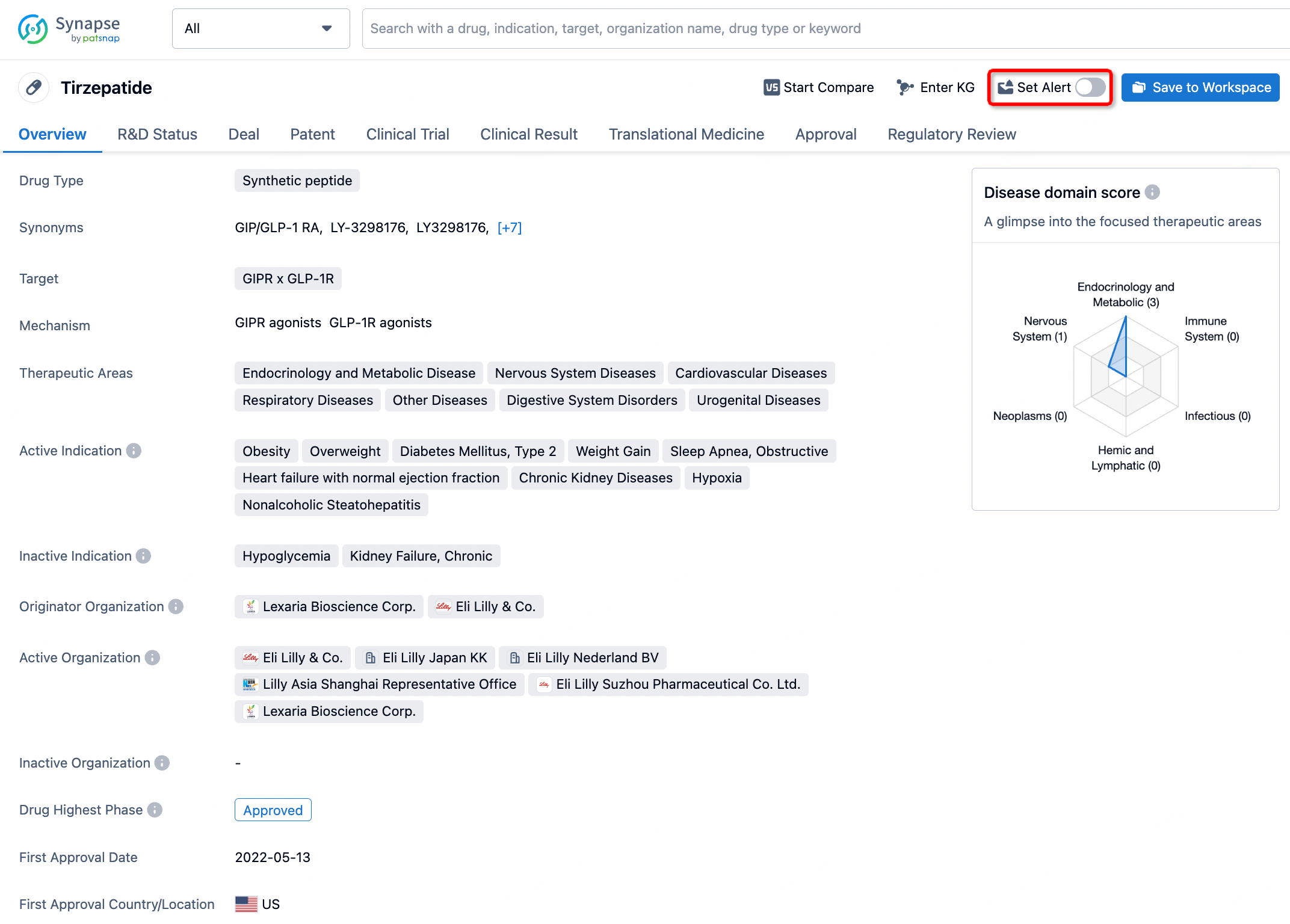
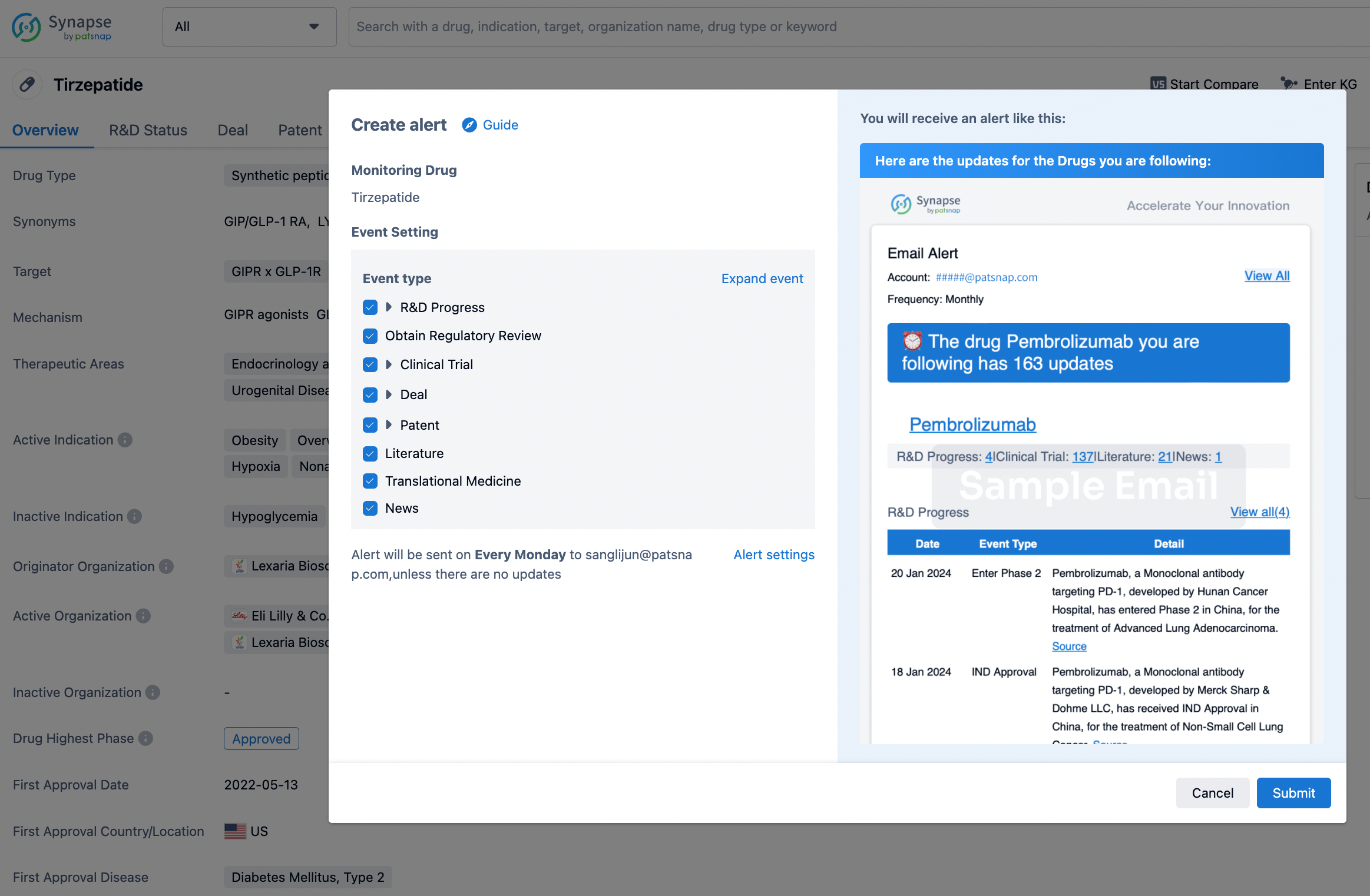
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!