Japan sanctions VYLOY™ (zolbetuximab) by Astellas as a new therapeutic for stomach cancer management
Astellas Pharma Inc. revealed that the Japanese Ministry of Health, Labour and Welfare has sanctioned the use of VYLOY™ (zolbetuximab), a therapeutic agent targeting anti-claudin 18.2 (CLDN18.2) for the treatment of individuals diagnosed with advanced, inoperable, or metastatic stomach cancer that tests positive for CLDN18.2. This marks the inaugural authorization worldwide of a CLDN18.2-specific medical treatment by a health regulatory body.
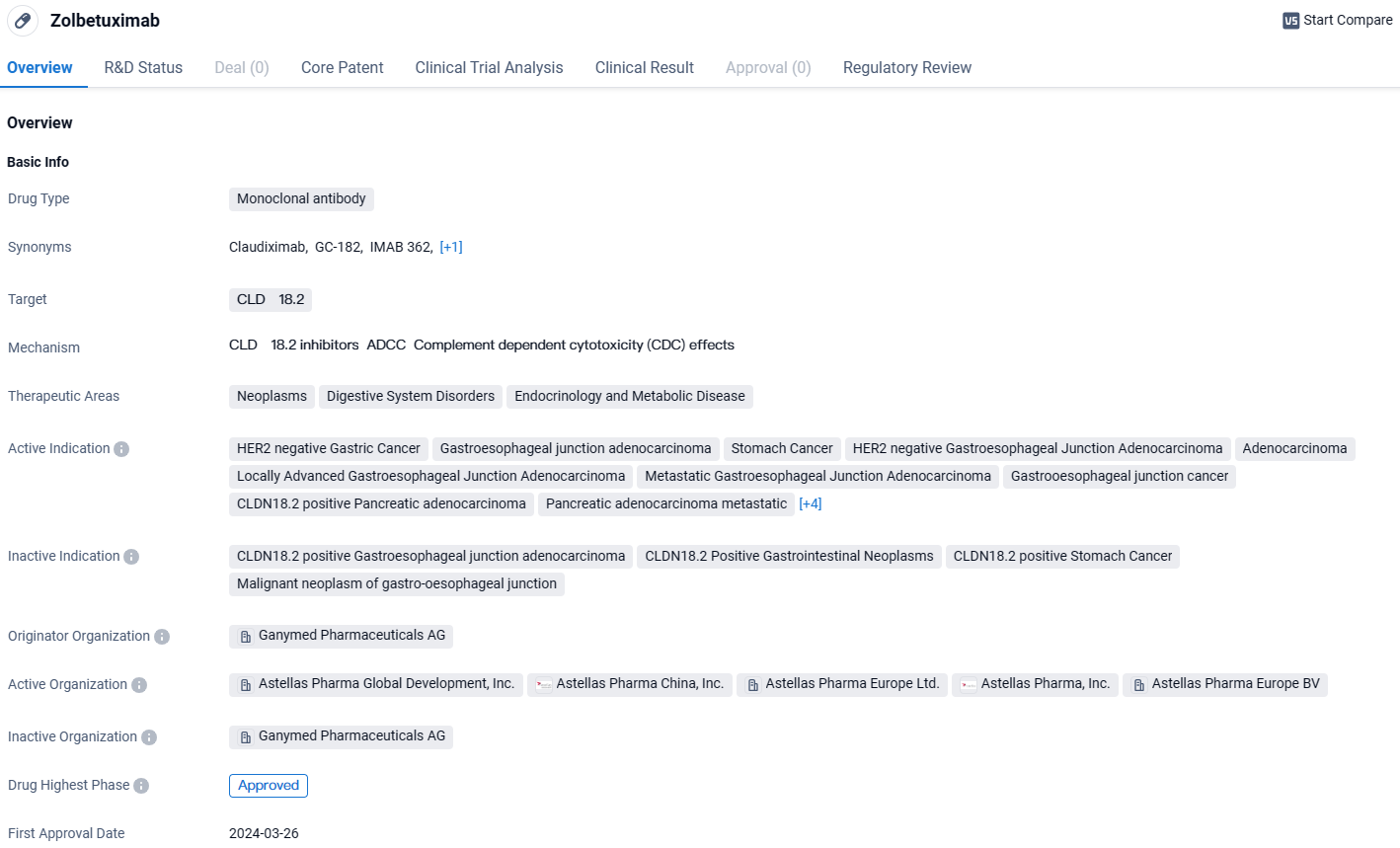
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Stomach cancer often goes unnoticed until it reaches later stages or begins spreading due to initial symptoms that are easily confused with more commonplace gastrointestinal issues. In 2022, Japan recorded 126,724 new cases, making it the nation's third most lethal form of cancer.
Dr. Moitreyee Chatterjee-Kishore, holding the position of Senior Vice President and directing Immuno-Oncology Development at Astellas, commented, "Receiving the green light from the MHLW for VYLOY is a milestone in managing stomach cancer, representing the singular personalized treatment approach for those afflicted with CLDN18.2-positive gastric cancer. Astellas is committed to fulfilling the dire need for an effective therapeutic approach for this aggressive cancer, especially prominent in Japan."
The Lead Researcher of the SPOTLIGHT Trial, Dr. Kohei Shitara, MD, emphasized, "With a scarce selection of specific treatments for late-stage gastric adenocarcinoma, pioneering new treatments is imperative. The findings support the use of VYLOY as a promising therapy for the CLDN18.2-positive demographic in Japan, which saw nearly 44,000 deaths due to this type of cancer in the year 2022."
In their quest to battle this disease, Astellas has joined forces with Roche Diagnostics to endorse the VENTANA CLDN18 RxDx Assay, a tailored immunohistochemistry test designed to find patients who could benefit from treatment with VYLOY. The test is initially available through several central labs in Japan, with expectations to broaden its availability as time progresses.
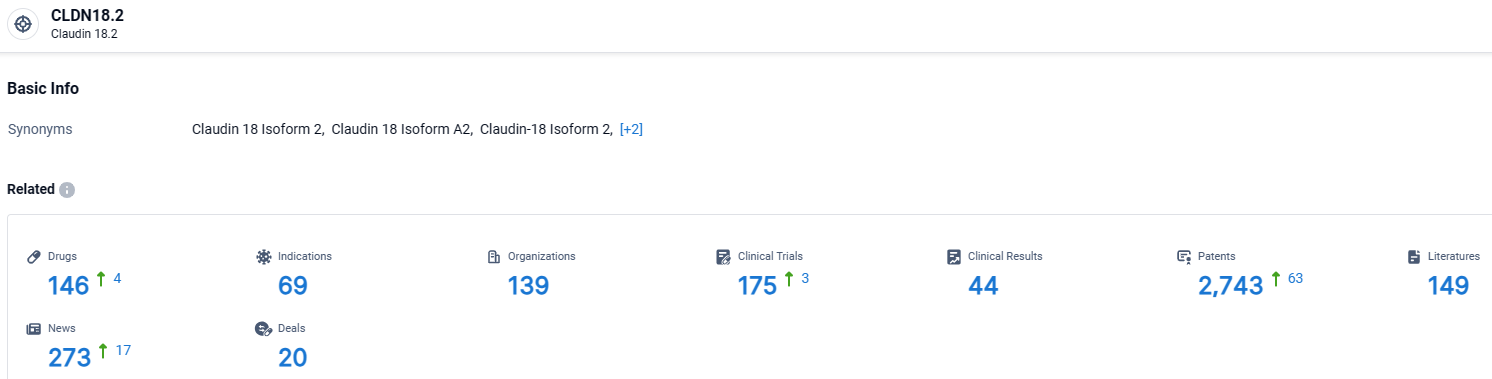
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 28, 2024, there are 146 investigational drugs for the CLDN18.2 target, including 69 indications, 139 R&D institutions involved, with related clinical trials reaching 44, and as many as 2743 patents.
Zolbetuximab's approval, target specificity, and therapeutic potential position it as a promising drug in the field of biomedicine. Further research and development, as well as market expansion efforts, could contribute to its success in addressing the needs of patients with various cancers and digestive system disorders.