JCR Pharmaceuticals Initiates Phase I Trial of JR-441 for Mucopolysaccharidosis Type IIIA
JCR Pharmaceuticals Co., Ltd. (TSE 4552; referred to as "JCR") has revealed that it has commenced dosing the first patient in Japan for the Phase I clinical trial of JR-441. This agent is an experimental enzyme replacement therapy aimed at treating mucopolysaccharidosis type IIIA (also known as Sanfilippo syndrome type A). JR-441 is a proprietary recombinant form of heparan N-sulfatase that has the ability to penetrate the blood-brain barrier (BBB).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
MPS IIIA is a rare hereditary condition marked by pronounced symptoms affecting the central nervous system (CNS), and there are presently no approved therapies available. Preliminary research has indicated that JR-441 could potentially alleviate CNS-related symptoms linked to MPS IIIA.
This open-label, multicenter, single-arm trial is designed to evaluate the safety, biological effects, and pharmacokinetics of JR-441 in individuals between the ages of 1 and 18 years diagnosed with MPS IIIA.
“MPS IIIA presents considerable challenges in managing CNS symptoms,” commented Dr. Kimitoshi Nakamura, Professor of Pediatrics at the Graduate School of Medical Sciences, Kumamoto University, and the study's medical expert. “This innovative treatment strategy signifies a new chapter in the management of this disorder. We have been looking forward to the chance to provide this therapy, and I am optimistic that its efficacy will be validated in clinical practice, ultimately enhancing the quality of life for both patients and their families.”
JR-441 has been granted Orphan Drug Designation by both the European Commission (EC) and the U.S. Food and Drug Administration (FDA), with a Phase I/II clinical trial currently ongoing in Germany that commenced in 2023 (JR-441-101, NCT06095388).
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
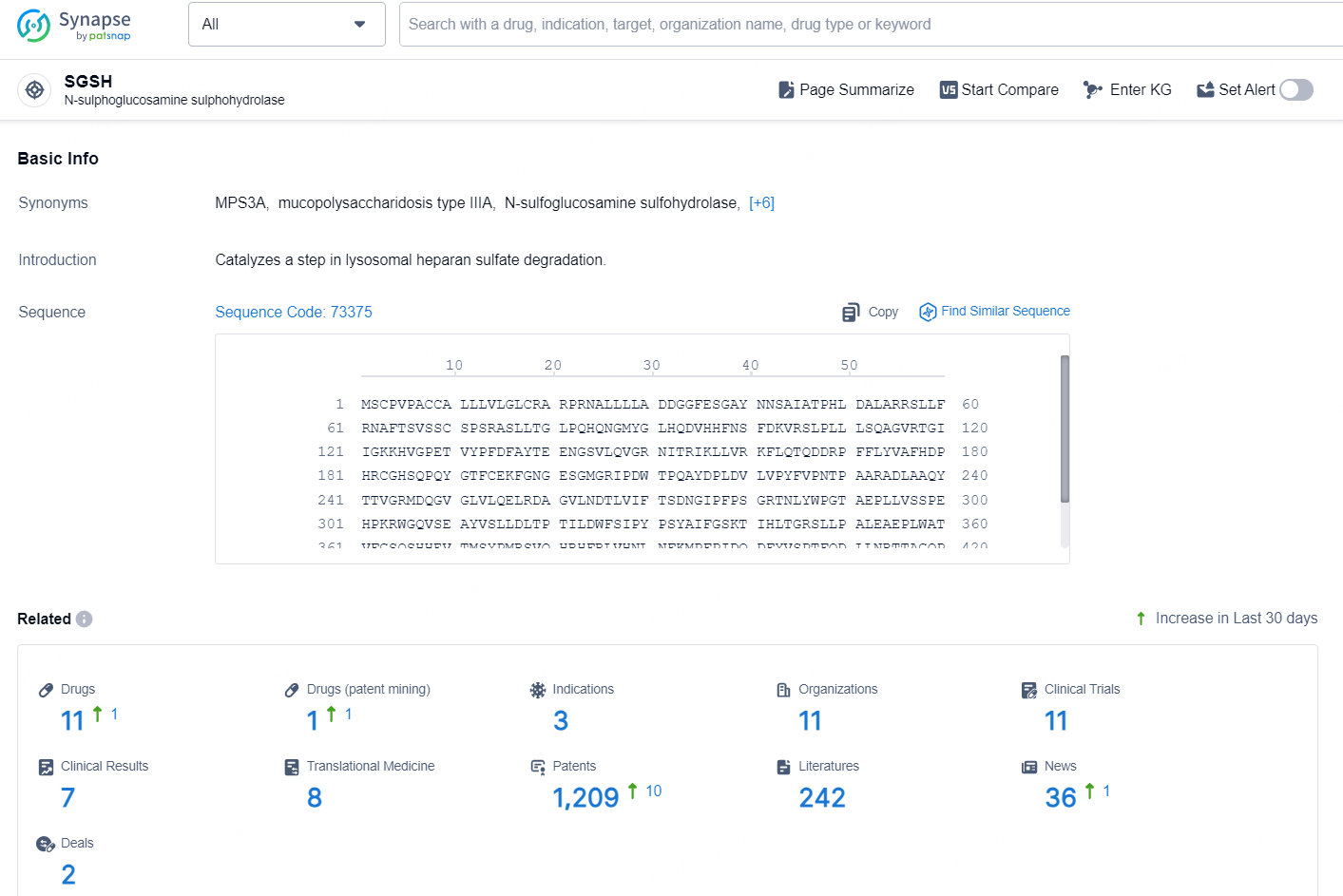
According to the data provided by the Synapse Database, As of November 5, 2024, there are 11 investigational drugs for the SGSH target, including 3 indication, 11 R&D institutions involved, with related clinical trial reaching 11, and as many as 1209 patents.
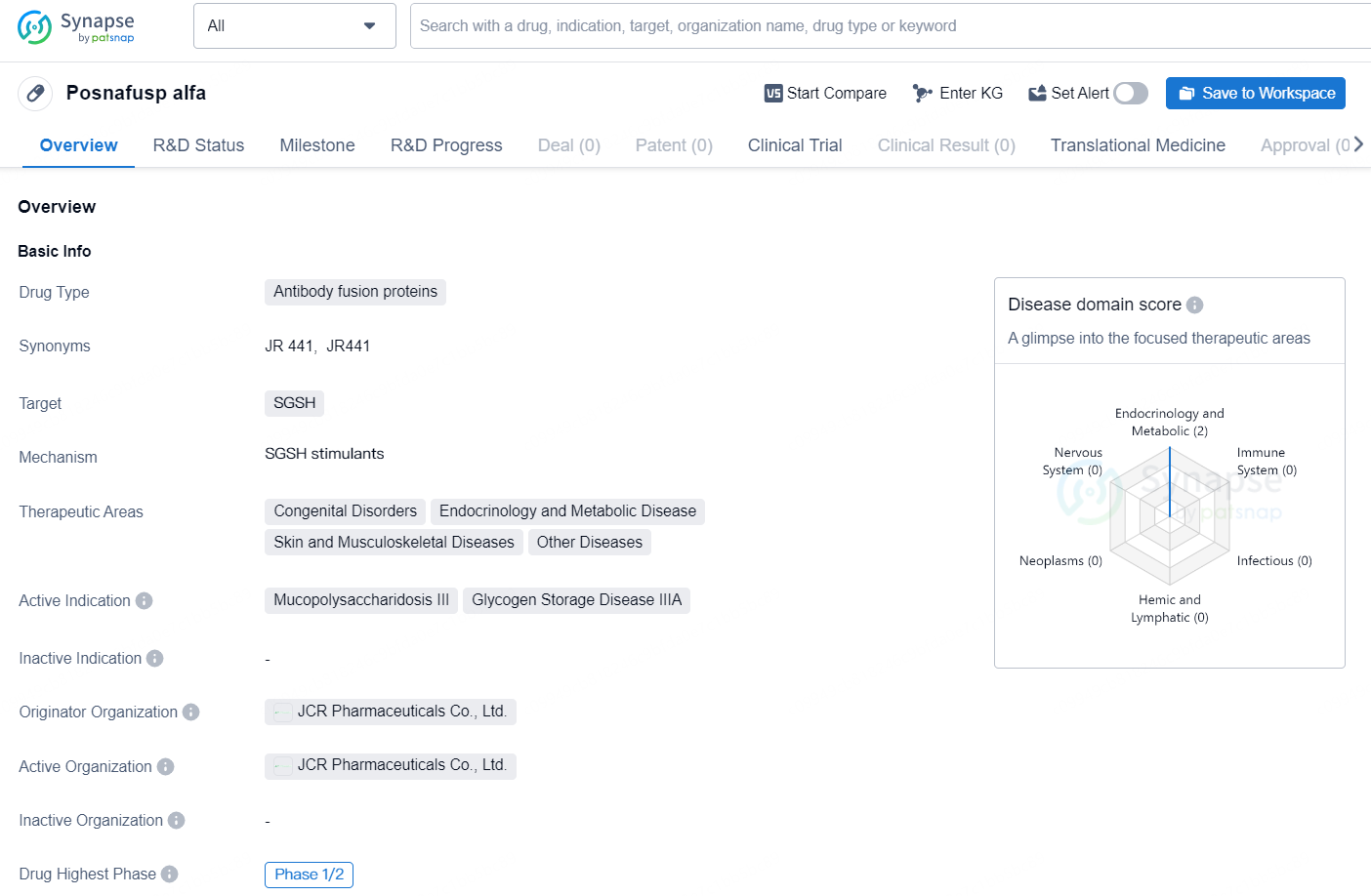
Posnafusp alfa is a drug classified as an antibody fusion protein, designed to target SGSH. The therapeutic areas of focus for this drug include congenital disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, as well as other diseases. The active indications for Posnafusp alfa are Mucopolysaccharidosis III and Glycogen Storage Disease IIIA.