JCR Pharmaceuticals Initiates Phase III Trial of JR-142 in Japan, a Prolonged-Release Growth Hormone
JCR Pharmaceuticals Co., Ltd. has reported that the initial patient has received treatment in the Phase III clinical trial of JR-142 (INN: redalsomatropin alfa) in Japan. This event signifies an important advancement in the progression of this novel therapy. JR-142 is a long-acting growth hormone treatment currently being evaluated for its efficacy in children with growth hormone deficiency.
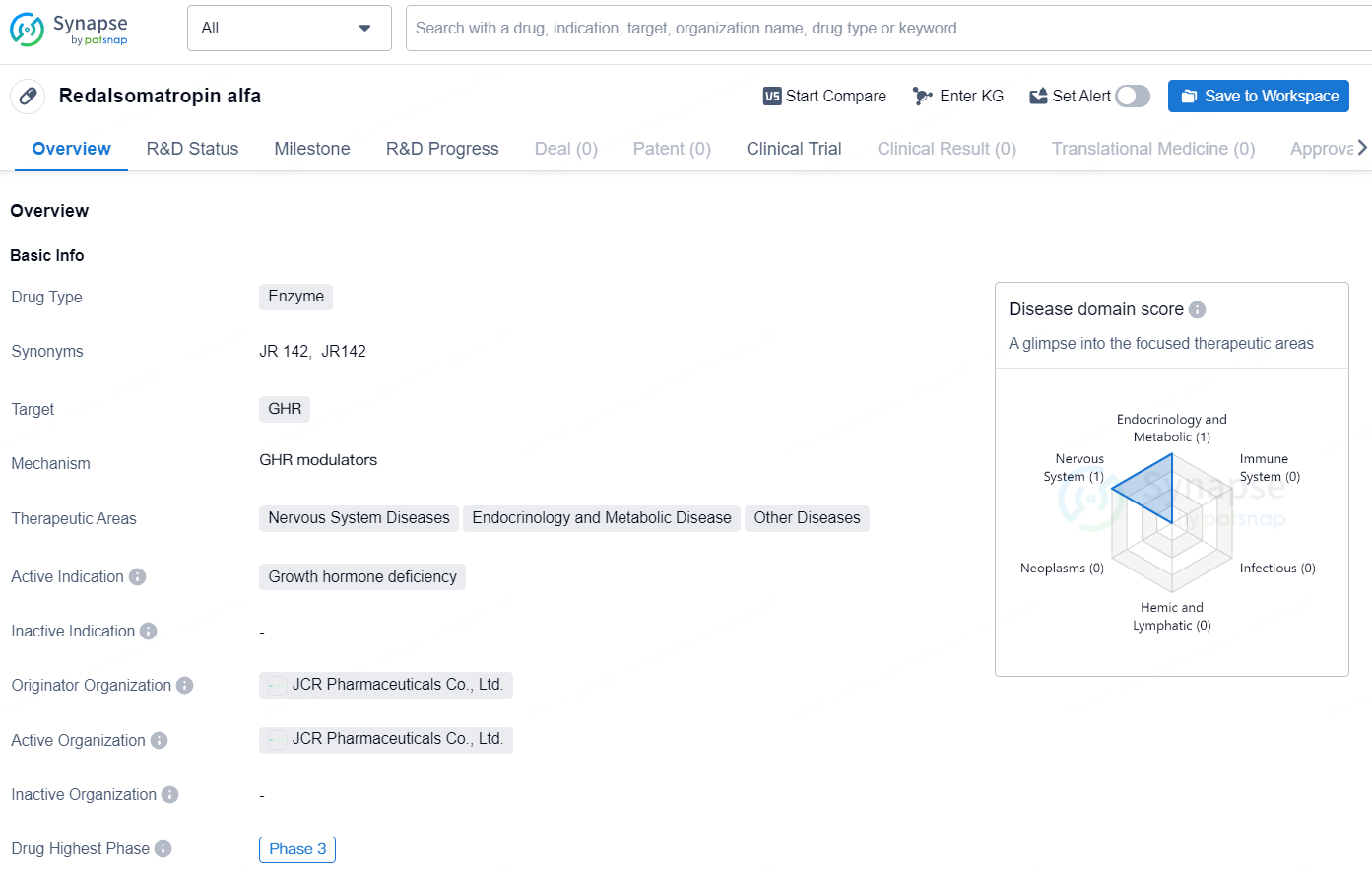
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The study involves 54 children and aims to assess the effectiveness of JR-142 in comparison to JCR's current offering, Growject®. Participants will undergo treatment for a period of 52 weeks, with growth outcomes being the primary indicator of success. Comprehensive details regarding the trial can be found in the Japan Register of Clinical Trials (jRCT2031240282).
“I am excited to witness advancements in the development of therapies for pediatric patients,” stated Professor Noriyuki Namba from the Division of Pediatrics and Perinatology at Tottori University Faculty of Medicine and a medical expert for the investigation. “Conventional growth hormone therapy necessitates daily injections; however, JR-142, with its long-acting properties, presents the option for once-weekly administration. This progress has the potential to significantly relieve the strain on both children and their families, enhancing both convenience and the overall quality of care while upholding rigorous safety standards.”
JCR is dedicated to the field of growth hormones, ensuring a reliable supply of high-quality pharmaceuticals and broadening treatment alternatives to accommodate the varied needs of patients.
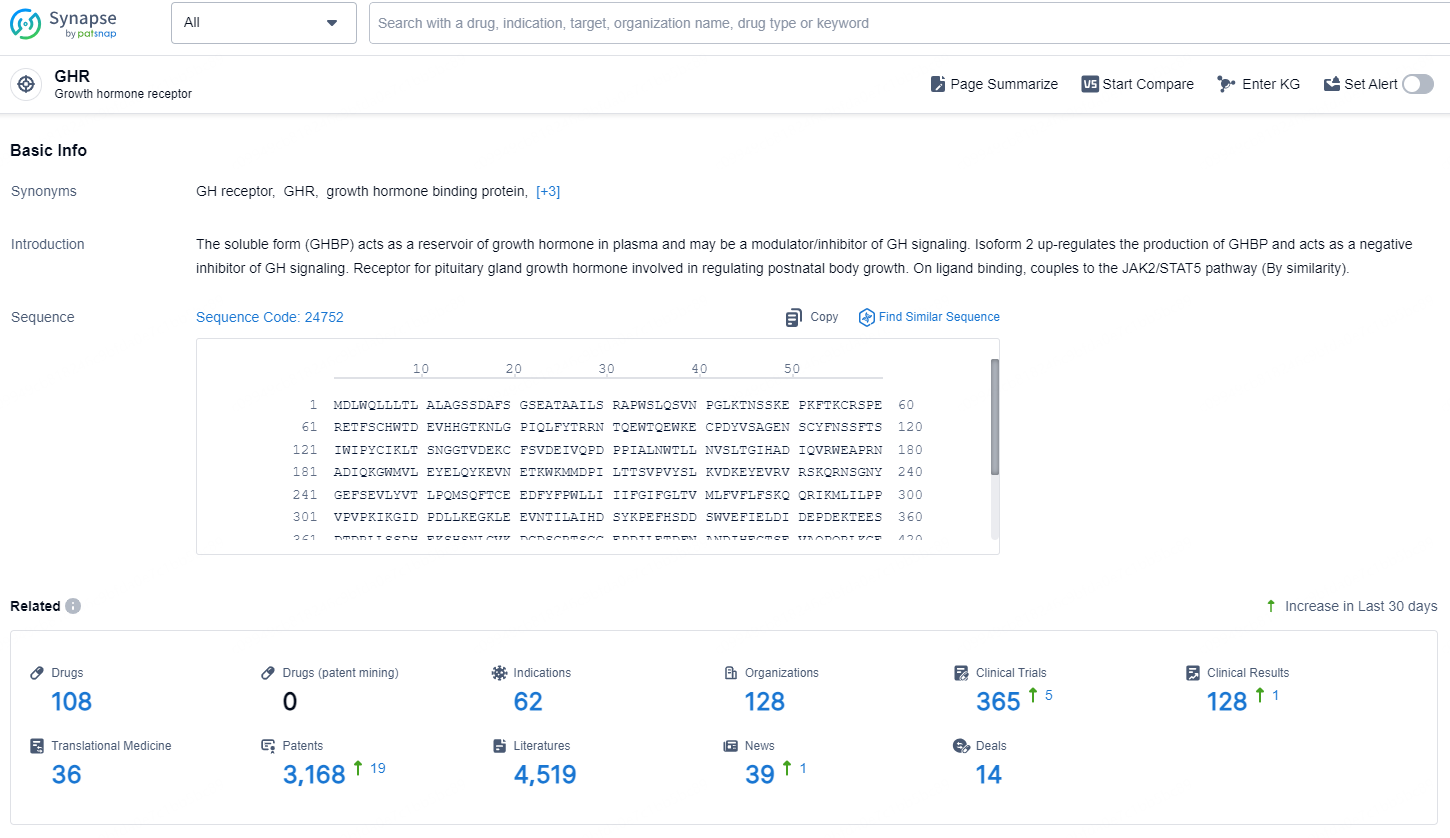
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 26, 2024, there are 108 investigational drug for the GHR targets, including 62 indications, 128 R&D institutions involved, with related clinical trials reaching 365, and as many as 3168 patents.
Redalsomatropin alfa is a type of drug classified as an enzyme. It primarily targets the growth hormone receptor (GHR) and is indicated for the treatment of growth hormone deficiency. The therapeutic areas in which Redalsomatropin alfa is being investigated include Nervous System Diseases, Endocrinology and Metabolic Disease, and Other Diseases.