Merck Secures Global License for Dual-Target Bispecific Antibody LM-299
Merck (NYSE: MRK), referred to as MSD in regions outside of the United States and Canada, has confirmed the completion of an exclusive global licensing deal for LM-299, an innovative investigational PD-1/VEGF bispecific antibody, with LaNova Medicines Ltd. As indicated in earlier communications, Merck is set to oversee the development, production, and marketing of LM-299.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Merck plans to account for a pre-tax expense related to the upfront payment of $588 million, roughly $0.18 per share, in the company’s GAAP and non-GAAP financial outcomes for the fourth quarter of 2024. Additionally, LaNova stands to gain up to $2.7 billion in milestone payouts that are contingent upon the technology transfer, development, regulatory approval, and commercialization of LM-299 across various indications, which includes an initial $300 million upon the expected completion of the technology transfer in 2025.
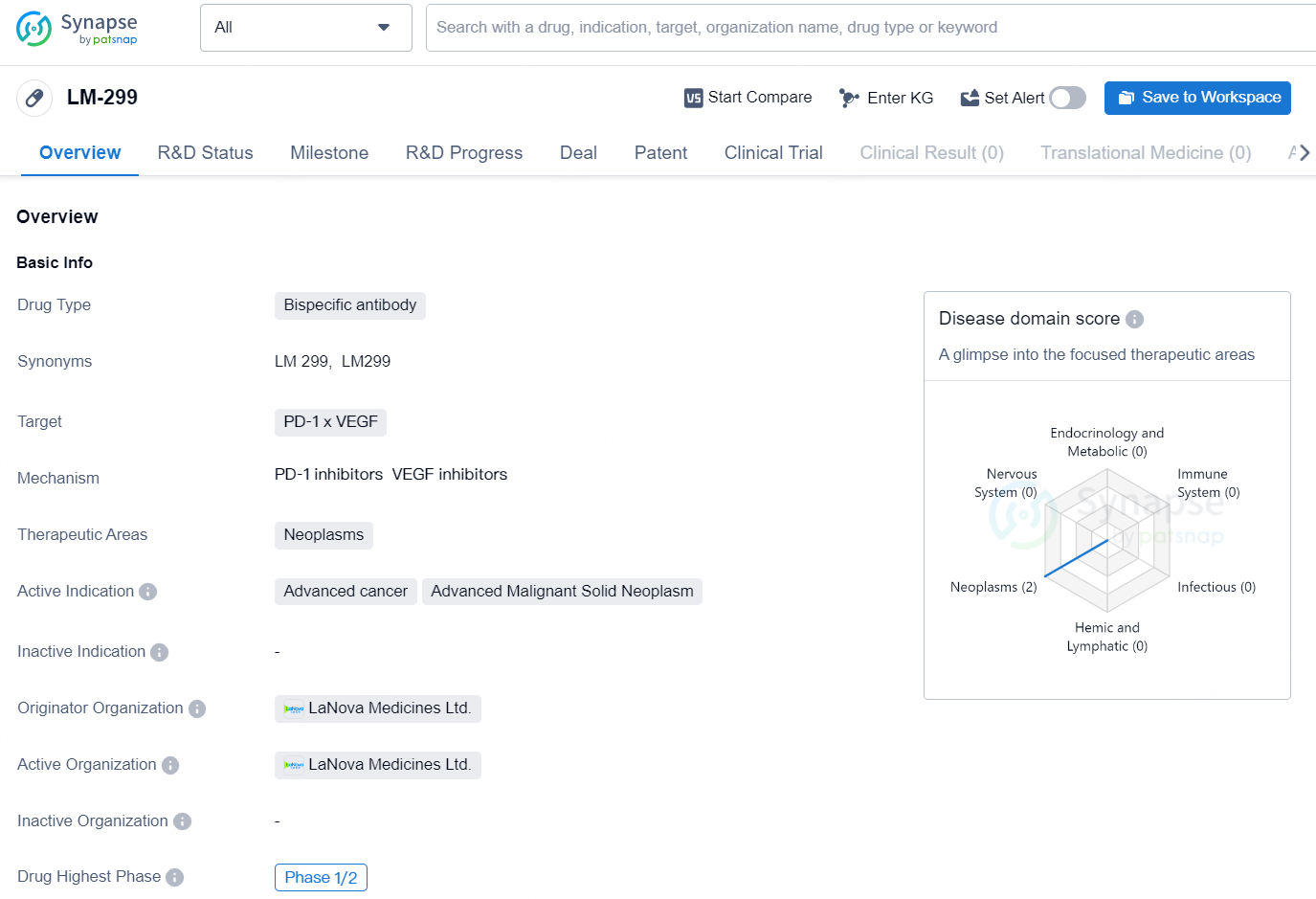
LM-299 is a novel investigational bispecific antibody aimed at simultaneously targeting programmed cell death protein-1 (PD-1) and vascular endothelial growth factor (VEGF). This cutting-edge therapeutic strategy seeks to disrupt both PD-1/PD-L1 and VEGF/VEGFR signaling pathways, effectively releasing a vital immune checkpoint while also preventing new blood vessel formation (angiogenesis). The molecular structure of LM-299 is distinct, featuring an anti-VEGF antibody conjugated with two C-terminal single domain anti-PD-1 antibodies. Currently, a Phase 1 clinical trial for LM-299 is actively recruiting patients in China.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
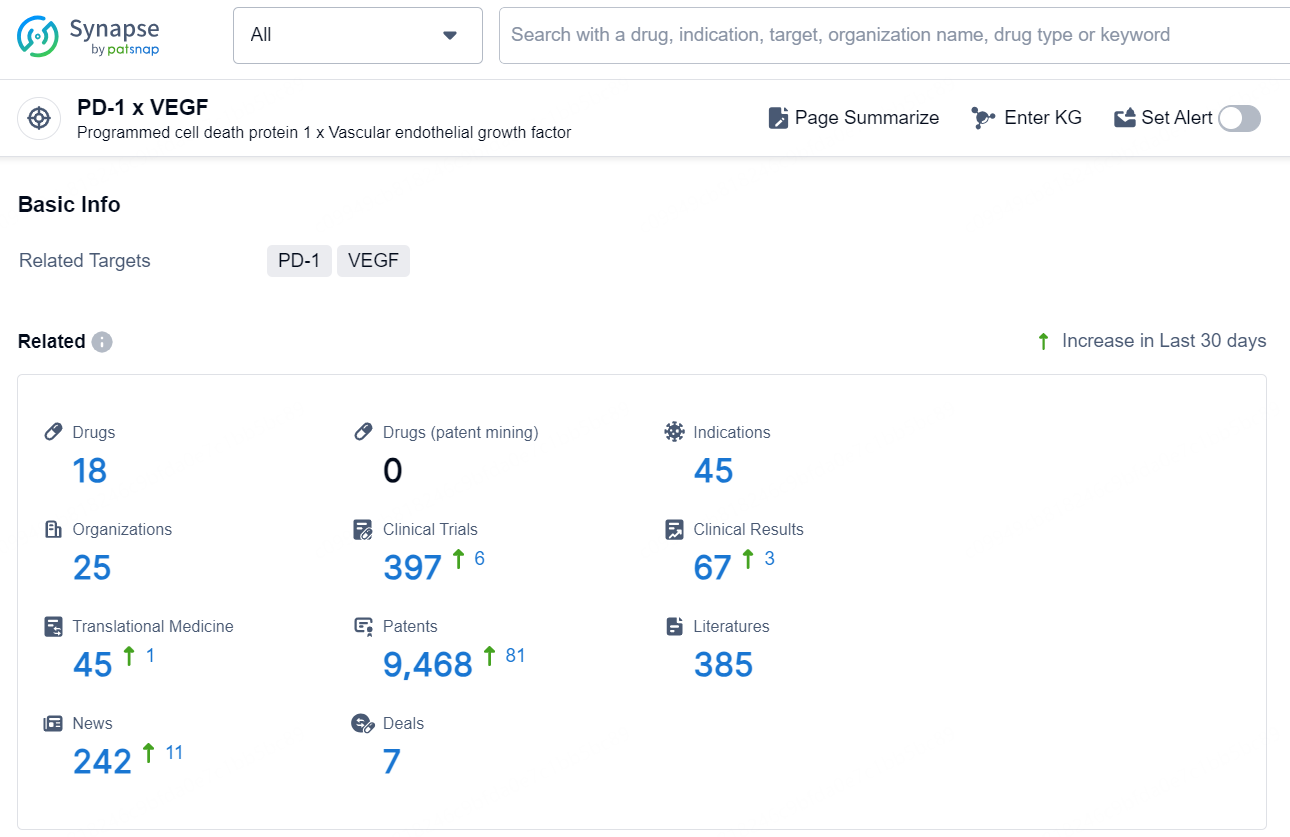
According to the data provided by the Synapse Database, As of December 26, 2024, there are 18 investigational drugs for the PD-1 x VEGF target, including 45 indications, 25 R&D institutions involved, with related clinical trials reaching 397, and as many as 9468 patents.
LM-299 is a bispecific antibody drug developed by LaNova Medicines Ltd. The drug targets the PD-1 x VEGF pathways and is intended for the treatment of neoplasms, specifically advanced cancer and advanced malignant solid neoplasms. As of the latest available information, LM-299 has reached the highest phase of clinical development, which is Phase 1/2 globally, and Phase 1/2 in China. This suggests that the drug has completed early-phase trials and is currently undergoing mid-phase clinical testing in both global and Chinese markets.