JCR Pharmaceuticals Reveals Findings from Phase I/II Research of JR-171 in people with Mucopolysaccharidosis Type I
JCR Pharmaceuticals Co., Ltd. has disclosed critical findings from the midway 52-week data of its global research phase I/II involving JR-171 (lepunafusp alfa) in patients diagnosed with mucopolysaccharidosis type I. JCR's proprietary J-Brain Cargo® technology has been deployed to formulate JR-171, which is a variant of recombinant α-L-iduronidase that holds the capability to penetrate the blood-brain barrier.
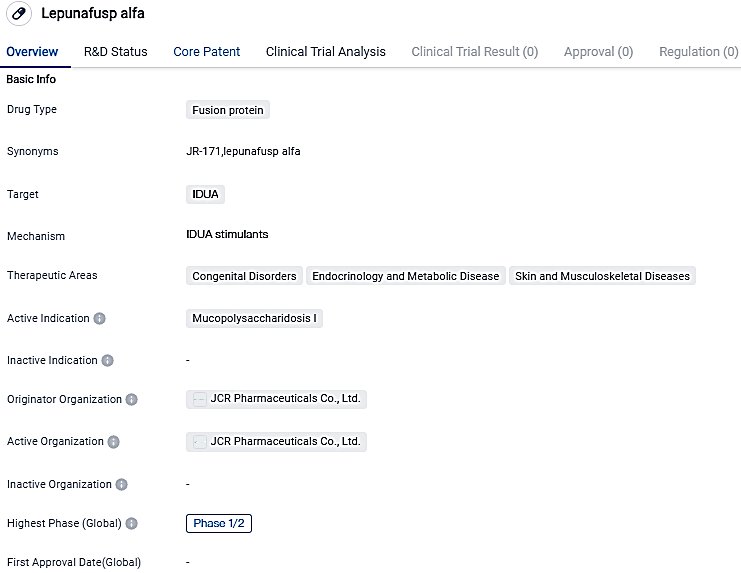
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The overall safety findings suggest that JR-171 is appropriate for the sustained treatment of individuals diagnosed with MPS I. Despite the study not being designed to prove impacts on CNS symptoms, initial data hint at JR-171's potential for delivering positive effects on neurological disease manifestations in symptomatic individuals.
For individuals previously exposed to α-L-iduronidase, somatic biomarkers remained constant, while showing a significant decrease in an untreated subject. This points to JR-171's capacity to match the somatic disease management of somatic enzyme replacement therapy. Substrate concentrations in the cerebrospinal fluid, a potential marker of substrate reduction influence on the CNS, notably decreased in all patients.
"It's reassuring to note other alterations in areas like communication, executive functionality, and other aspects crucial in everyday life for those treated with JR-171, apart from meeting the primary and secondary targets," commented Paul Harmatz, MD, researcher at UCSF Benioff Children's Hospital Oakland. "Based on these findings, JR-171 shows promise in addressing both somatic and neurological manifestations of MPS I, which is the ultimate needed."
JR-171 is a manmade fusion protein of an antibody targeted at the human transferrin receptor and α-L-iduronidase, the enzyme that is either absent or malfunctioning in subjects with MPS I. Being projected to cross the blood-brain-barrier via transferrin receptor mediated transcytosis, it's anticipated to counteract central nervous system indications and symptoms of the disease thus addressing a significant gap in the treatment of MPS I. The US FDA had previously awarded Fast Track status to JR-171.
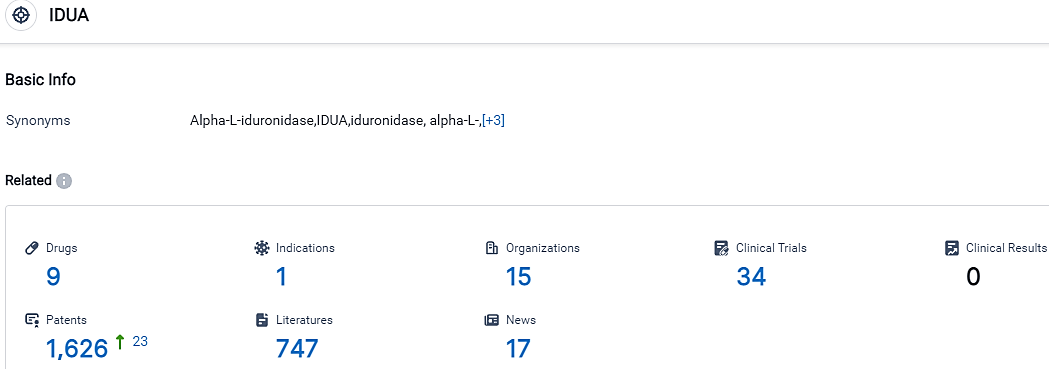
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 9, 2023, there are 9 investigational drugs for the IDUA target, including 1 indications, 15 R&D institutions involved, with related clinical trials reaching 34,and as many as 1626 patents.
Lepunafusp alfa targets the IDUA enzyme and is primarily focused on treating Mucopolysaccharidosis I. The drug also shows potential in addressing other congenital disorders, endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. While it is still in Phase 1/2 of clinical trials, Lepunafusp alfa has shown promise and further research is needed to determine its effectiveness and safety.