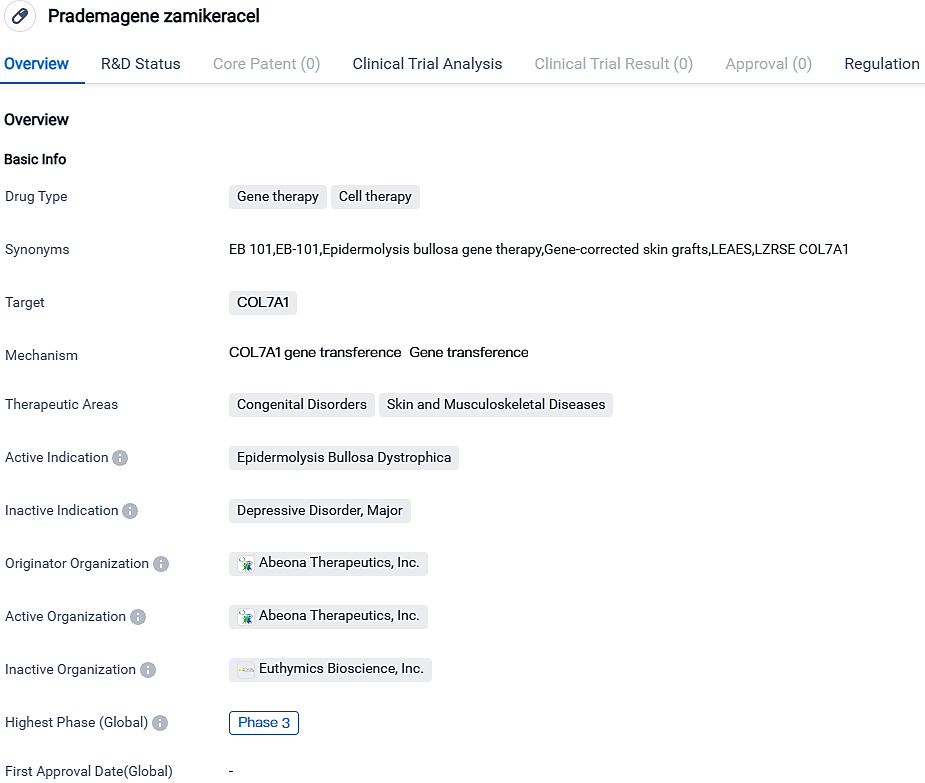
Abeona Therapeutics has requested a U.S. FDA Biologics License for expedited review of EB-101, a treatment for Recessive Dystrophic Epidermolysis Bullosa patients
Abeona Therapeutics Inc. has reported that they've lodged a BLA with U.S. FDA with a request to endorse EB-101, their experimental autologous, modified cell treatment, aimed at treating patients suffering from recessive dystrophic epidermolysis bullosa. With this application, Abeona expressed a desire for Priority Review, which if accepted, would reduce the FDA’s review timeline to half a year, starting from BLA's filing approval date, in contrast to the standard analysis period of 10 months.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Abeona's submission of the BLA for EB-101 is a groundbreaking achievement and a pivotal step toward ensuring our experimental EB-101 product becomes an alternative for RDEB patients as the premier personalized cell therapy capable of providing lasting aid in wound healing and pain management from a single use." Abeona's CEO, Vish Seshadri, commented.
“We are thankful for the FDA's level of involvement and valuable insight during the months approaching the pre-BLA meeting. My appreciation also extends to the entire submission team for their relentless dedication and hard work in finalizing Abeona’s inaugural BLA submission,” Vish Seshadri further stated.
The filing of the BLA for EB-101 was a result of continuous dialogues with the FDA, backed by clinical effectiveness and safety data derived from the crucial Phase 3 VIITAL™ study, and reinforced by evidence from a Phase 1/2a study. Outcomes from the VIITAL™ study were revealed at the inaugural meeting of the International Societies for Investigative Dermatology in May 2023. Longitudinal data of up to eight years, and quality of life data from the Phase 1/2a study, were published in the Orphanet Journal of Rare Diseases.
The FDA typically makes a verdict on BLA acceptance within 60 days of submission. Provided it's accepted with Priority Review, Abeona anticipates potential BLA approval in Q2, 2024. At that juncture, Abeona envisages it could qualify for a Priority Review Voucher. The voucher, if conferred, could be utilized by the business to expedite the review of a future BLA or New Drug Application, or it may be sold to another entity.
EB-101 is a patient-specific, engineered cell therapy presently in development for addressing recessive dystrophic epidermolysis bullosa, a rare disorder of connective tissue with no known cure. Therapy with EB-101 involves utilizing gene transfer to introduce the functioning COL7A1 gene into a subject's personal skin cells and subsequently retransplant them to the patient.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
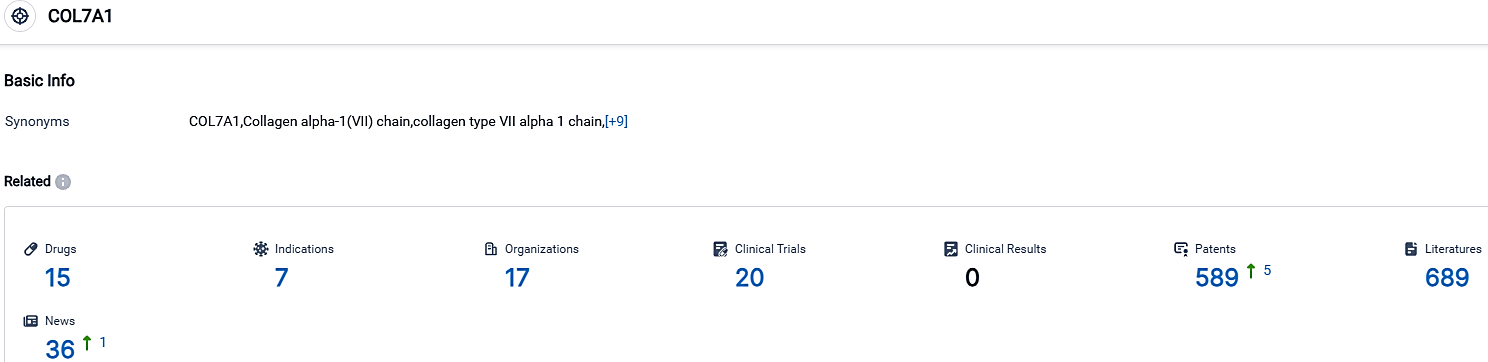
According to the data provided by the Synapse Database, As of October 3, 2023, there are 15 investigational drugs for the COL7A1 target, including 7 indications,17 R&D institutions involved, with related clinical trials reaching 20,and as many as 589 patents.
The U.S. FDA has bestowed EB-101 with various accolades such as Regenerative Medicine Advanced Therapy, Breakthrough Therapy, Orphan Drug, and Rare Pediatric Disease designations. Abeona is responsible for the production of EB-101 for the VIITAL study, carried out in its fully-fledged gene and cell therapy manufacturing unit based in Cleveland, Ohio. FDA has yet not granted approval for EB-101 as it remains as a product undergoing investigation.