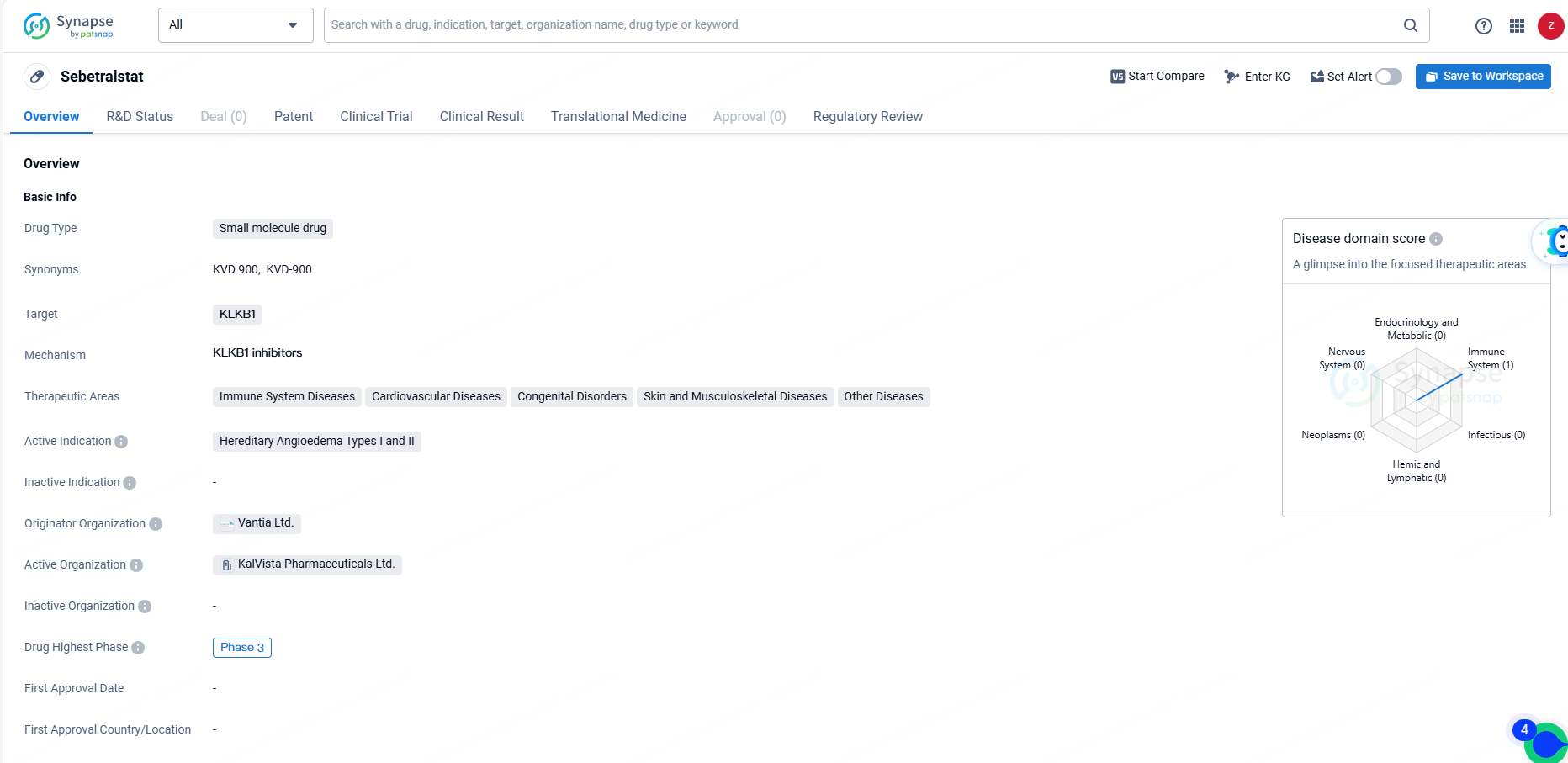
KalVista Seeks FDA Approval for Sebetralstat, First Oral Treatment for Hereditary Angioedema
KalVista Pharmaceuticals, Inc. has disclosed that they have filed a New Drug Application with the U.S. Food and Drug Administration for the evaluation of sebetralstat. This innovative investigational oral plasma kallikrein inhibitor is intended for the immediate treatment of hereditary angioedema attacks in both adult and pediatric populations aged 12 and above.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Our NDA submission signifies a critical juncture not just for our organization, but for the entire HAE community, as we endeavor to introduce a groundbreaking therapeutic innovation through the first-ever oral, on-demand treatment for HAE," stated Ben Palleiko, Chief Executive Officer of KalVista.
"This milestone reflects the extensive effort and dedication of the entire KalVista team, bolstered by the indispensable support from individuals living with HAE, our collaborations with the HAE scientific community, the HAEA and HAEi patient advocacy groups, regulators, and other stakeholders. This underscores our unwavering commitment to addressing the persistent unmet needs of those afflicted by this rare condition. Should approval be granted, we anticipate that sebetralstat could serve as a cornerstone therapy that will revolutionize the way HAE is managed," Palleiko continued.
The NDA submission is grounded in previously reported clinical trial outcomes, notably data from the KONFIDENT phase 3 trial and the KONFIDENT-S extension trial. In the phase 3 trial, sebetralstat met the primary endpoint, with both 300 mg and 600 mg doses providing significant symptom relief more rapidly than placebo. The median time to initiation of symptom relief was shorter compared to placebo.
Aligned with earlier studies, sebetralstat exhibited a favorable safety profile, comparable to placebo, with no serious treatment-related adverse events recorded. This advantageous safety profile has been consistently observed in all clinical trials for sebetralstat up to this point.
The FDA has a 60-day review period to decide whether the NDA is complete and ready for review. KalVista expects to receive a decision from the FDA regarding the submission's status in September. The Company also plans to submit additional marketing authorization applications to other global health authorities throughout 2024.
If approved, sebetralstat would mark the first oral, on-demand therapy for individuals with HAE. The NDA submission includes patients aged 12-17 years, a demographic that faces significant challenges with injectable treatments. KalVista intends to initiate a pediatric trial in the third quarter of 2024, which, if successful, would enable future applications to extend coverage to patients aged 2-11 years.
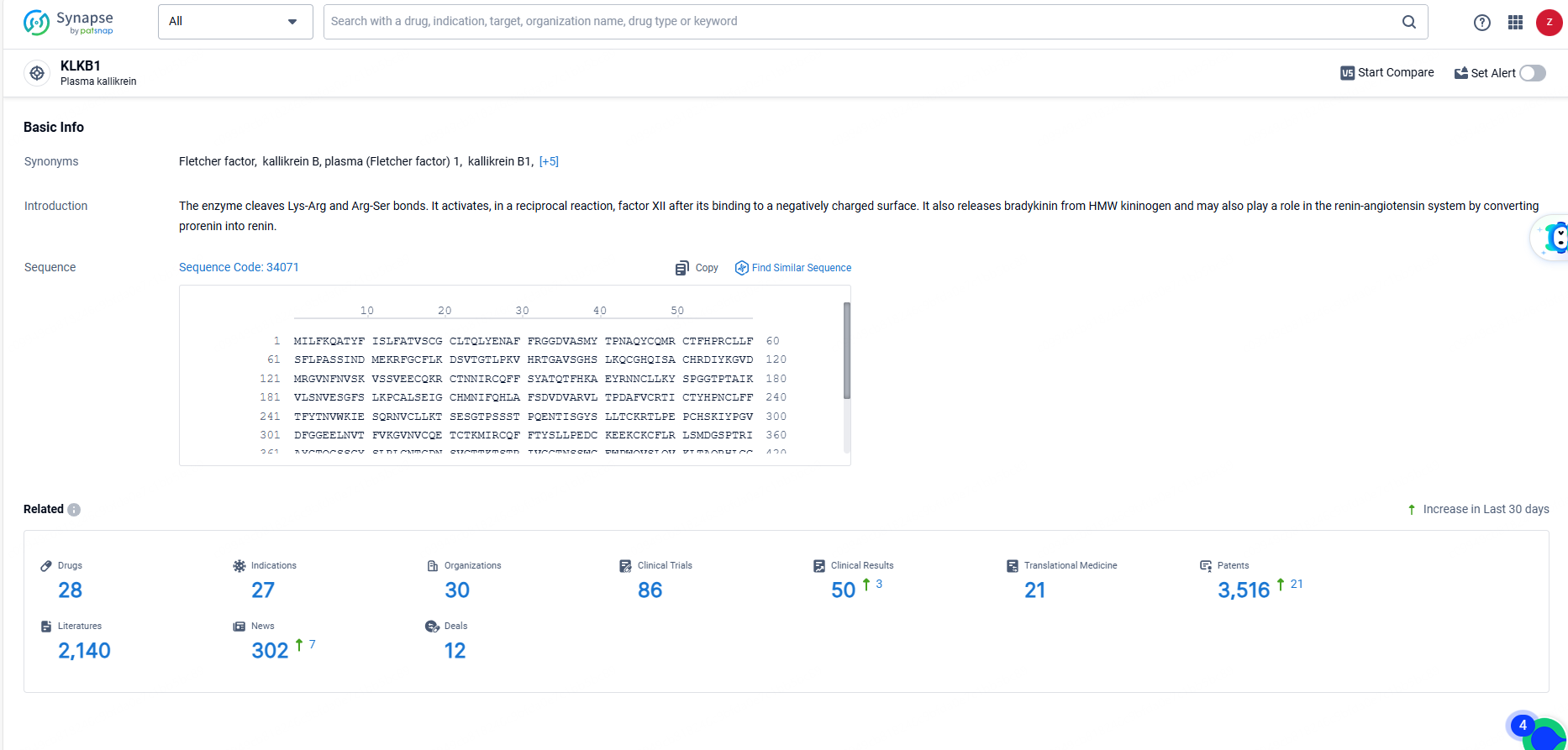
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 21, 2024, there are 28 investigational drugs for the KLKB1 targets, including 27 indications, 30 R&D institutions involved, with related clinical trials reaching 86, and as many as 3516 patents.
Sebetralstat's small molecule nature, its targeting of KLKB1, its therapeutic areas, and its regulatory status collectively position it as a promising candidate for the treatment of Hereditary Angioedema and other related conditions. As the drug continues through the development process, further clinical data will be essential in determining its ultimate impact on patient outcomes.