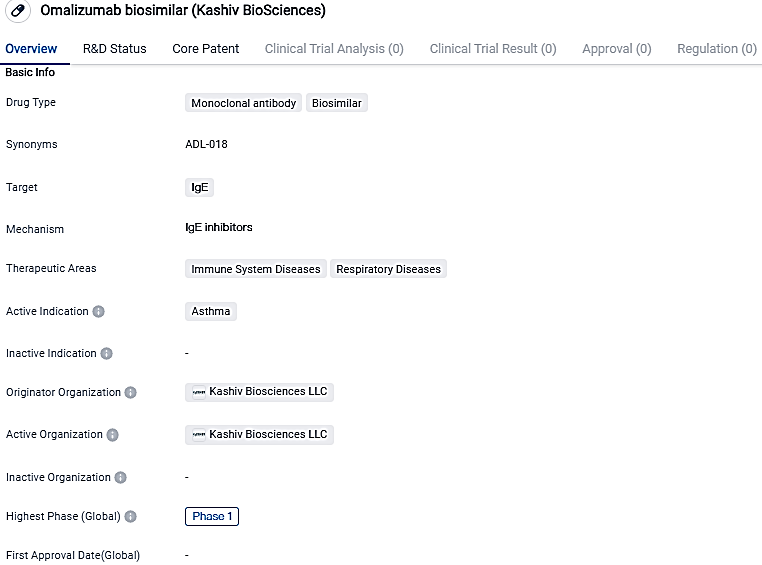
Kashiv BioSciences Administers First Patient in Phase III Clinical Trial for ADL018
Kashiv BioSciences, LLC declared the start of enrollment for their Phase III clinical trial initiating with their first patient for their biosimilar candidate, ADL018, a potential alternative to XOLAIR®. Their aim in conducting this study is to compare how ADL018 and XOLAIR® will perform in terms of effectiveness, safety, tolerability, and degree of immune response in patients suffering from chronic idiopathic/spontaneous urticaria and who continue to show symptoms even with H1 antihistamine treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study is set to include 600 patients worldwide across various locations including the United States, Europe, and India.
Dr. Sandeep Gupta, Kashiv's CEO, said, "We're excited to communicate the commencement of our international Phase III trial for ADL018, following the first patient's enrollment. It marks a significant stride towards introducing Kashiv's third biosimilar into the market, reinforcing our commitment to comprehensive research, development, and manufacturing. Our concentration on high-quality and economical development steers us towards progressing our extensive selection of reasonably priced biosimilars, impacting patients across the globe positively."
Dr. Chandramauli Rawal, Kashiv's COO, continued, "Launching this Phase III trial underpins our ceaseless dedication to cultivating top-grade biosimilars. Additionally, it acknowledges the immeasurable commitment and groundwork from our skilled team. This key study's successful execution is something we trust in, and we anticipate more recruitment and regular patient tracking."
ADL018, developed as a biosimilar counterpart to XOLAIR® (omalizumab), is a humanized monoclonal antibody. It works by preventing the bond of IgE with the high-affinity IgE receptor on mast cells and basophils' exterior. It's prescribed for those above 12 suffering from Chronic Spontaneous Urticaria, whose hives remain unresolved with H1 antihistamine treatment.
Also, omalizumab is approved for people above 6 with moderate to severe, persistent asthma whose symptoms aren't controlled well with inhaled corticosteroids; and those above 18 with chronic rhinosinusitis with nasal polyps.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
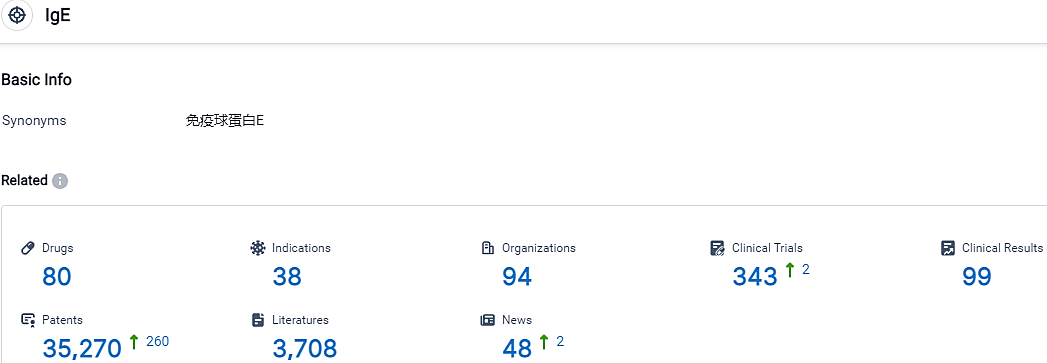
According to the data provided by the Synapse Database, As of October 10, 2023, there are 80 investigational drugs for the IgE target, including 38 indications, 94 R&D institutions involved, with related clinical trials reaching 343,and as many as 35270 patents.
Omalizumab is a monoclonal antibody drug targeting IgE. It is being developed as a biosimilar to provide a more affordable alternative for the treatment of asthma, a chronic respiratory disease. ADL018 has the same pharmaceutical form, dosage strength, route of administration and dosing regimen as US and EU-approved omalizumab. Global sales of XOLAIR® in the last twelve months preceding June 30, 2023 were about $3.7 billion.