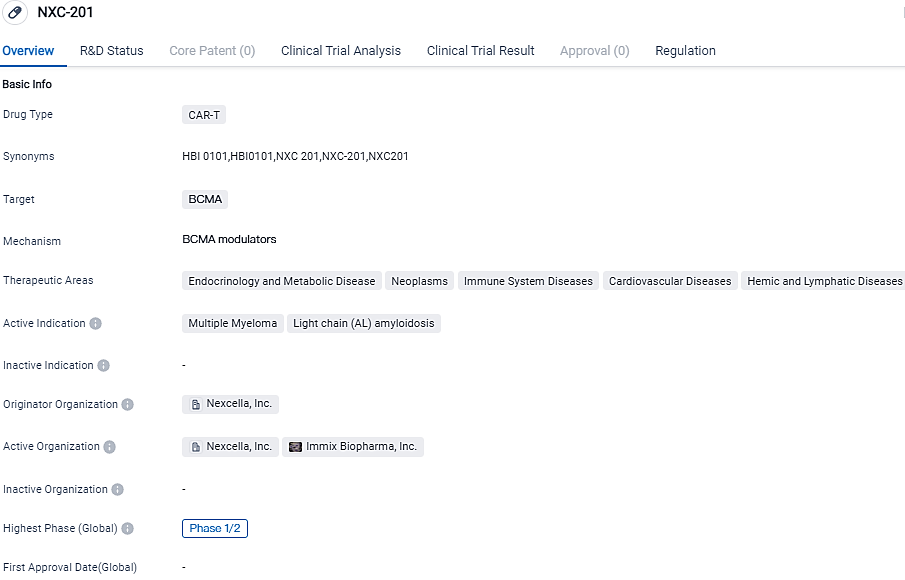
Nexcella reveals clinical data from NXC-201 study in Multiple Myeloma
Nexcella Inc. shared new information from its current Phase 1b/2 NEXICART-1 trial of the company's original, self-sourced, BCMA-directed chimeric antigen receptor T cell treatment NXC-201. New data was collected from 63 patients suffering from multiple myeloma and was showcased during a poster exhibit at the International Myeloma Society's 20th Annual Gathering in Athens, Greece, held from September 27-30, 2023.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are greatly inspired by NXC-201's clinical findings," stated Polina Stepensky, M.D., who heads the Hadassah Medical Organization’s Department of Bone Marrow Transplantation and Immunotherapy for Adults and Children, and serves as the chief study investigator. "The general response rate results, in our opinion, are persuasive as 72% was the total response rate declared for ABECMA from its crucial 100-participant KarMMa examination in relapsed/refractory multiple myeloma."
NEXICART-1 is an ongoing Phase 1b/2a, single-arm research that assesses the safety and performance of NXC-201, in adults carrying relapsed/refractory multiple myeloma and relapsed/refractory AL amyloidosis.
The main objective of the Phase 1b segment of the research was to describe the safety and affirm the recommended Phase 2 dosage of NXC-201. The Phase 2 segment of the research will examine the efficacy and safety of NXC-201 with overall survival, progression-free survival, and response rates as per the International Myeloma Working Group Uniform Response Criteria as the outcomes.
Nexcella plans to submit data on multiple myeloma to the FDA once 100 patients have been administered NXC-201. The anticipated main outcome for NXC-201 in relapsed/refractory AL Amyloidosis is the general response rate. Nexcella is planning to put forward data for AL amyloidosis to the FDA after giving treatment to 30-40 patients with NXC-201.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
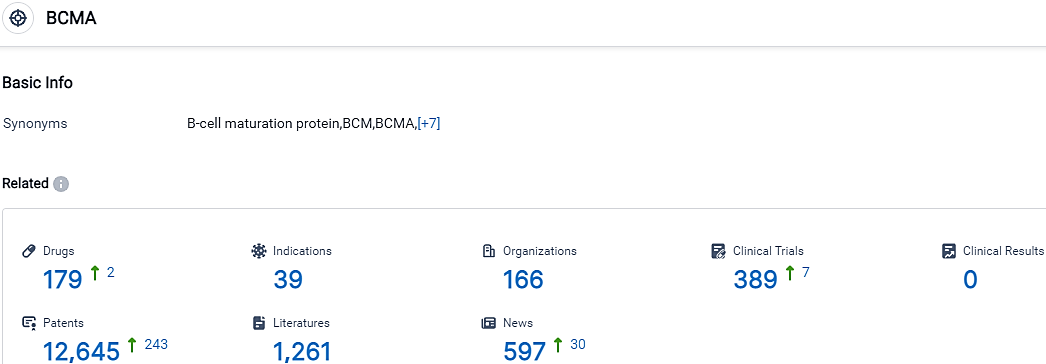
According to the data provided by the Synapse Database, As of October 9, 2023, there are 179 investigational drugs for the BCMA target, including 39 indications, 166 R&D institutions involved, with related clinical trials reaching 389,and as many as 12645 patents.
NXC-201 is a BCMA-targeted investigational chimeric antigen receptor T cell therapy that is being studied in a comprehensive clinical development program for the treatment of patients with relapsed or refractory multiple myeloma and AL amyloidosis. However, as it is still in the early stages of development, further research and clinical trials are needed to determine its safety and efficacy.