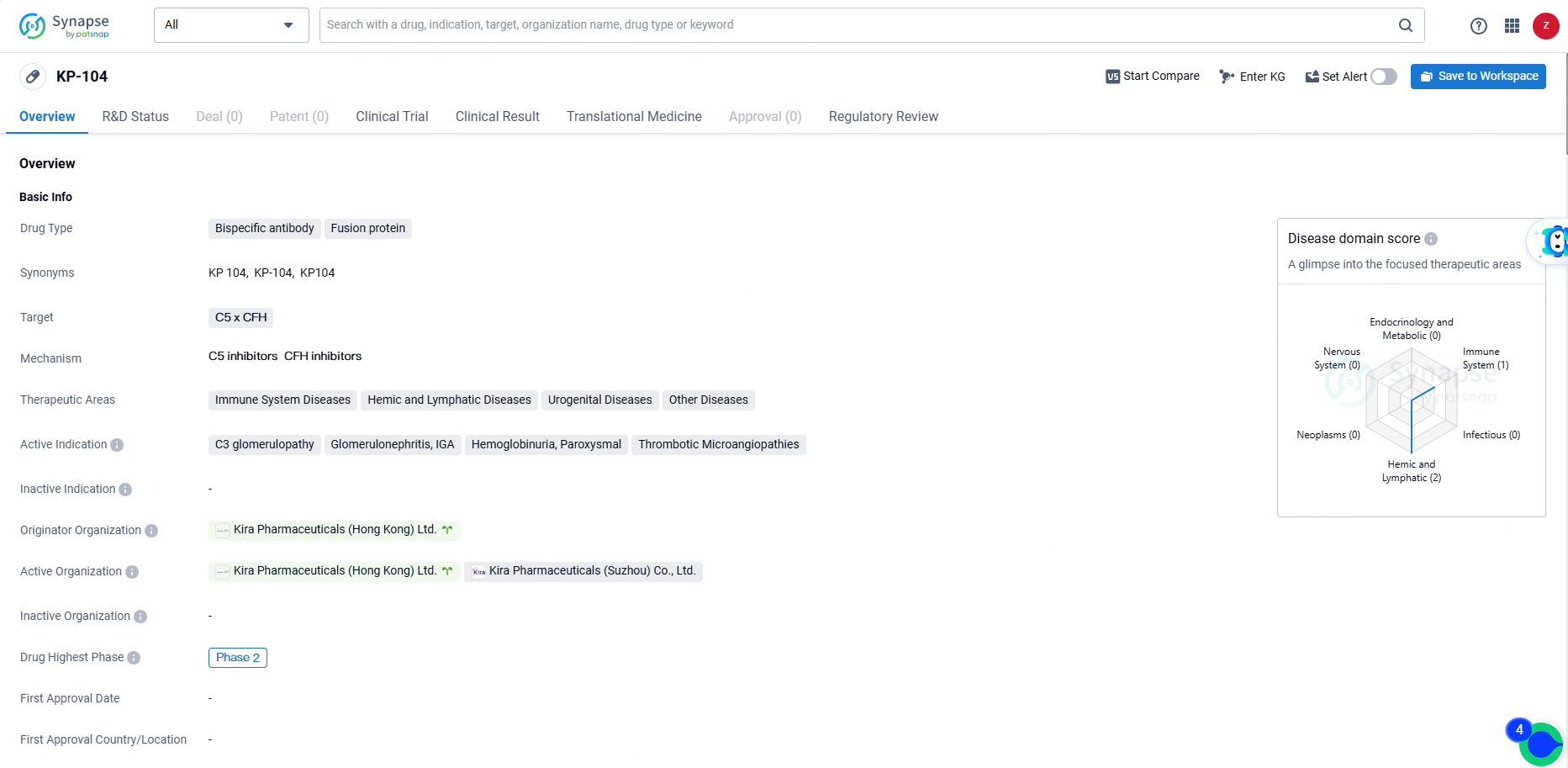
Kira Pharmaceuticals Presents Positive KP104 Phase 2 PNH Results at 2024 EHA Congress: Safety and Effectiveness
Kira Pharmaceuticals, an international biotechnology firm leading the way in revolutionary complement therapies for immune-mediated illnesses, has reported favorable long-term outcomes from its Phase 2 trial involving KP104 in complement inhibitor-naïve individuals with PNH. KP104 is a groundbreaking dual-targeting complement inhibitor (C5 & Factor H) addressing both the alternative and terminal pathways.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The results were shared at the 2024 European Hematology Association Hybrid Congress in Madrid, Spain, underscoring KP104’s groundbreaking potential as a new monotherapy for PNH to meet currently unmet medical needs.
Key findings from the research include data from 18 patients who received subcutaneous KP104 for up to 65 weeks, with 24-26 weeks of treatment at the Optimal Biological Dose for all participants, following a dose-escalation phase involving three cohorts.
None of the patients required RBC transfusions from the first day until the 65th week of KP104 treatment.
All secondary endpoints showed marked clinical improvements: normalization of absolute reticulocyte counts, bilirubin levels, and FACIT-fatigue scores after transitioning to OBD.
The promising long-term outcomes from the Phase 2 trial further illustrate KP104’s potential as an ideal first-line monotherapy for safely and effectively managing both intravascular and extravascular hemolysis in PNH. Global Phase 3 trials are being planned to potentially establish KP104 as the new standard of care for PNH.
“We are highly encouraged by the strong efficacy and positive safety profile exhibited by KP104 in our Phase 2 trial,” said Dr. Wenru Song, Head of R&D at Kira Pharmaceuticals, “these long-term results provide a solid foundation to advance KP104 into Phase 3 trials. We are dedicated and eager to deliver this promising treatment to patients suffering from PNH as swiftly as possible.”
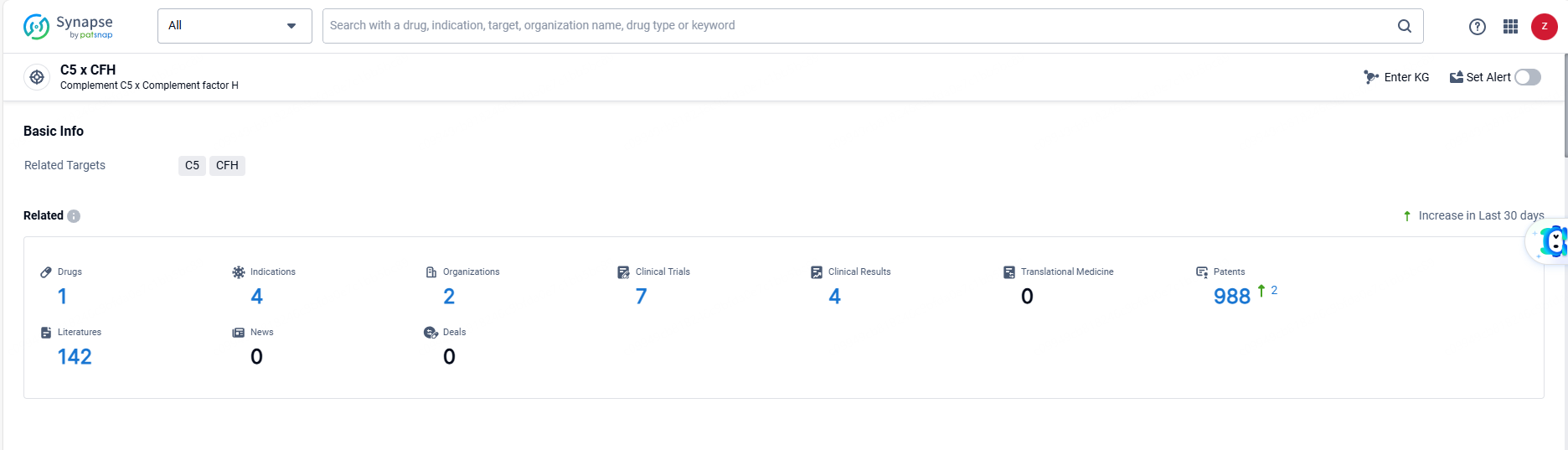
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 21, 2024, there are 1 investigational drugs for the C5 and CFH targets, including 4 indications, 2 R&D institutions involved, with related clinical trials reaching 7, and as many as 988 patents.
KP104 is a first-in-class bifunctional biologic designed to simultaneously block both the alternative (Factor H) and terminal (C5) complement pathways, providing a powerful and synergistic method of targeting the validated drivers of complement-mediated disease. KP104 has been granted Orphan Drug Designation by the FDA for the treatment of paroxysmal nocturnal hemoglobinuria. Phase 2 trials will be conducted globally, including in the U.S., China, and Australia. KP104 is an investigational agent not yet approved for any indication by any health authority.