Subcutaneous Amivantamab BLA Submitted to U.S. FDA for EGFR-Mutant Non-Small Cell Lung Cancer
Food and Drug Administration. This application is for a fixed-dose combination of amivantamab and recombinant human hyaluronidase meant for subcutaneous injection. It targets all the indications that are currently approved or under review for intravenous RYBREVANT (amivantamab-vmjw) in specific patients suffering from non-small cell lung cancer.
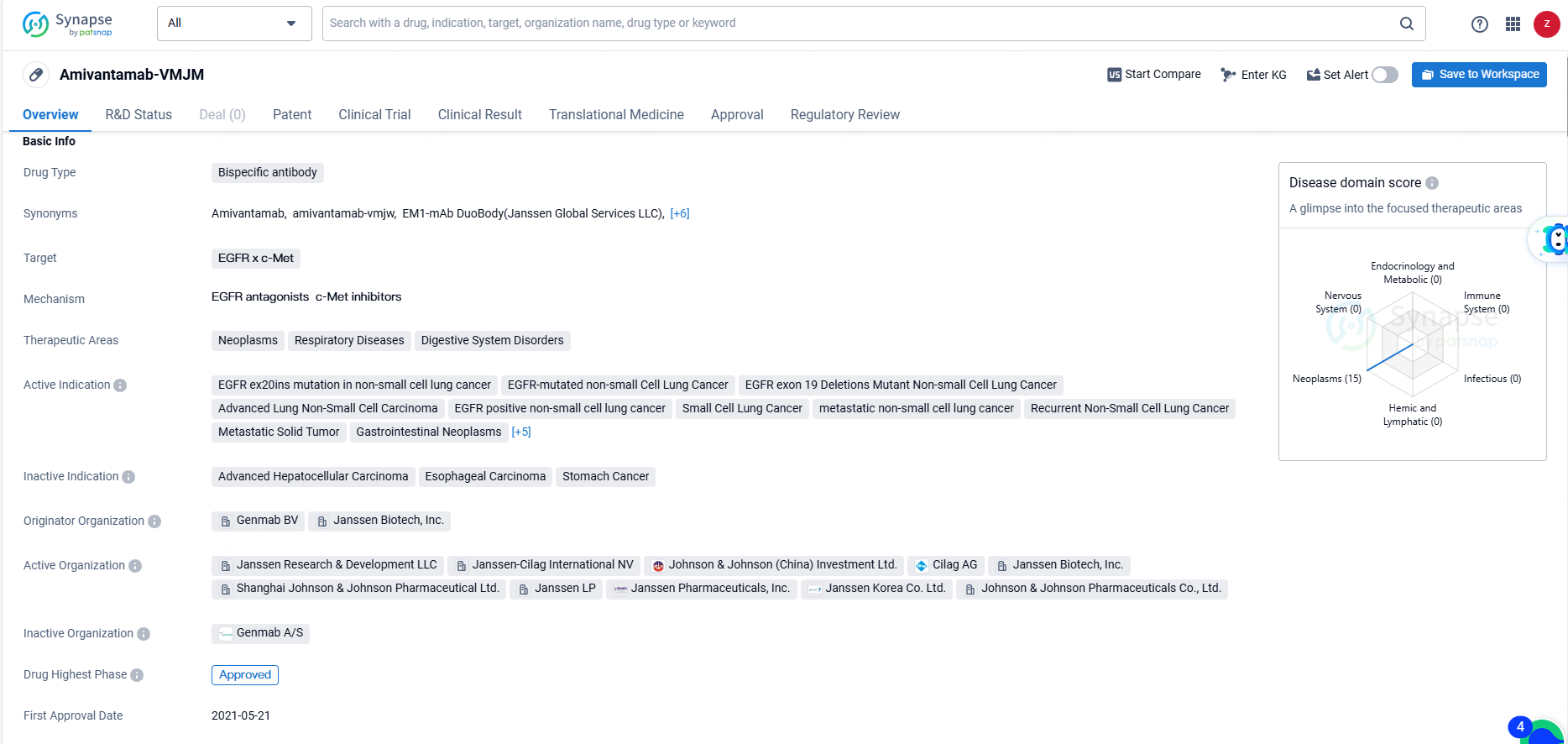
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 3 PALOMA-3 study data, disclosed at the 2024 American Society of Clinical Oncology Annual Meeting and published in the Journal of Clinical Oncology, highlighted that subcutaneous (SC) administration of amivantamab achieved overall response rates similar to its intravenous (IV) counterpart in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) exon 19 deletion or L858R mutations. SC amivantamab also boasted shorter administration times and a five-fold decrease in infusion-related reactions, in addition to superior overall survival, progression-free survival, and duration of response.
These efficacy outcomes are unprecedented in a study focused on IV and SC comparability. The Biologics License Application (BLA) submission features data from the Phase 2 PALOMA-2 study, which assessed SC amivantamab in scenarios where IV amivantamab was previously submitted for approval, supporting dosing schedules of every two and three weeks.
“RYBREVANT administered intravenously is essential for patients with EGFR-mutated NSCLC,” stated Kiran Patel, M.D., Vice President of Clinical Development for Solid Tumors at Johnson & Johnson Innovative Medicine. “This subcutaneous form, given in about five minutes, represents a significant advancement that could revolutionize the treatment experience for patients, oncologists, and nursing staff. We anticipate collaborating with the FDA and international regulators in reviewing these applications.”
This submission follows two significant recent achievements for the RYBREVANT IV formulation, namely, the FDA's approval of RYBREVANT combined with chemotherapy as the first-line treatment for NSCLC patients with EGFR exon 20 insertion mutations, based on the Phase 3 PAPILLON study, and a positive opinion from the CHMP for this combination therapy in Europe.
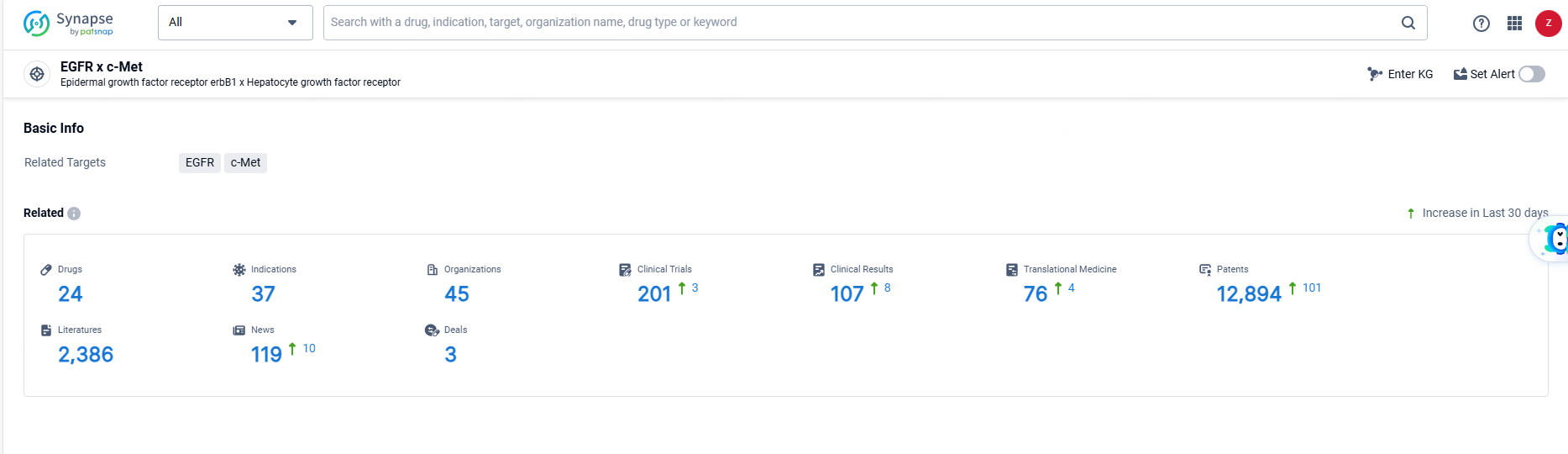
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 21, 2024, there are 24 investigational drugs for the EGFR and c-Met target, including 37 indications, 45 R&D institutions involved, with related clinical trials reaching 201, and as many as 12894 patents.
Amivantamab-VMJM is a bispecific antibody drug that targets the EGFR and c-Met proteins. It is used in the treatment of various neoplasms, respiratory diseases, and digestive system disorders.Amivantamab-VMJM represents a significant advancement in the treatment of various types of cancer, particularly lung cancer, and has the potential to improve outcomes for patients with these conditions. Its approval in the United States and ongoing regulatory processes in other countries indicate the drug's potential to make a meaningful impact on the pharmaceutical industry and patient care.