Kymera Therapeutics Reveals New Preclinical Data for KT-621, an Oral STAT6 Degrader, at ATS Conference
Kymera Therapeutics, Inc. revealed new preclinical findings for KT-621, a powerful and selective oral heterobifunctional degrader targeting STAT6, at the American Thoracic Society Annual Meeting in San Diego, California. The results showed that KT-621's efficacy in an asthma model was comparable to a high dose of the IL-4Rα antibody, dupilumab. Specifically, KT-621 effectively inhibited all tested cytokines, chemokines, and cell infiltrates associated with TH2 inflammation in asthma.
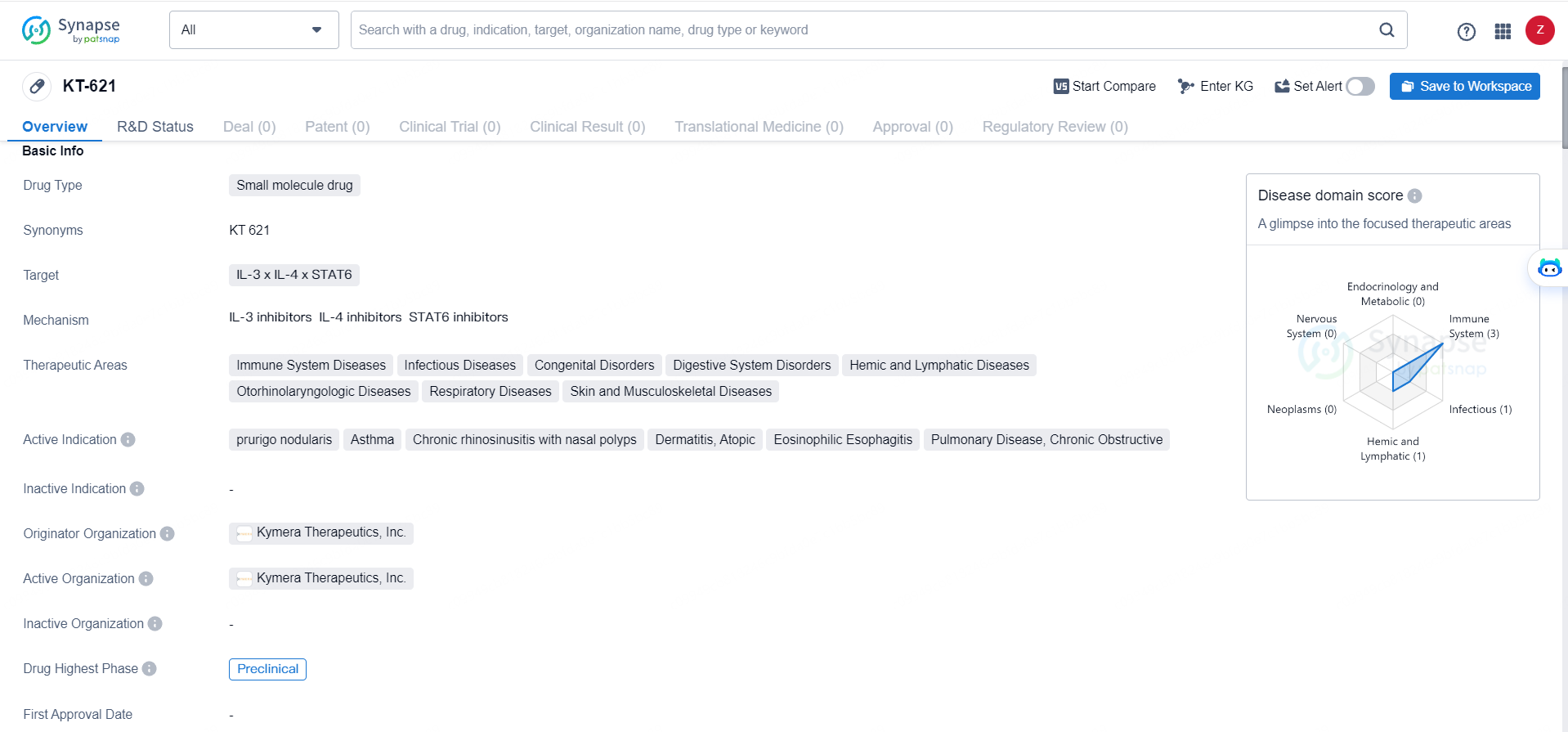
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Company has revealed additional histology results indicating that KT-621 taken orally in low, daily doses shows a similar improvement in lung remodeling as seen with dupilumab. These findings emphasize KT-621's promising profile as a potential oral therapy for asthma and other TH2-related respiratory conditions. Kymera plans to commence Phase 1 trials for KT-621 in the latter half of 2024 and anticipates reporting Phase 1 data in the first half of 2025.
“Having successfully introduced the first degrader for immunological diseases into the clinic with KT-474, our current objective is to develop further oral small molecule degraders with biologics-like efficacy to benefit the maximum number of patients,” stated Kymera Therapeutics’ Founder, President, and CEO, Nello Mainolfi, PhD.
"KT-621 shows promise in treating a range of TH2 immune-mediated disorders and could address the shortcomings of current treatments like biologics and conventional small molecule inhibitors. We believe KT-621 offers the convenience of a daily oral medication while potentially delivering efficacy akin to dupilumab in common allergic conditions such as asthma, which could revolutionize existing treatment approaches and broaden patient access," Dr. Mainolfi added.
In previous presentations, KT-621 demonstrated exceptional selectivity for STAT6 over other STATs and effectively inhibited IL-4/IL-13 activity in critical human TH2 cell assays with picomolar potency, outperforming dupilumab. Furthermore, preclinical studies revealed that low daily oral doses of KT-621 led to near-complete in vivo STAT6 degradation in disease-related tissues and were well tolerated.
At the ATS conference, new data was presented showing that in a model of intranasal house dust mite (HDM)-induced asthma in hIL4/hIL4RA humanized mice, daily oral doses of KT-621 administered for 30 days were well tolerated and exhibited significant efficacy comparable to a saturating dose of dupilumab targeting IL-4Rα. KT-621 effectively suppressed TH2 inflammation, including B cell activation, eosinophil recruitment, serum IgE, and HDM-specific IgG1 production, and reduced lung disease severity in this mouse model.
Overall, the preclinical data accumulated so far underscore KT-621's potential as a treatment for TH2 allergic diseases, offering best-in-pathway prospects due to its dupilumab-like efficacy and the convenience of an oral dosage form.
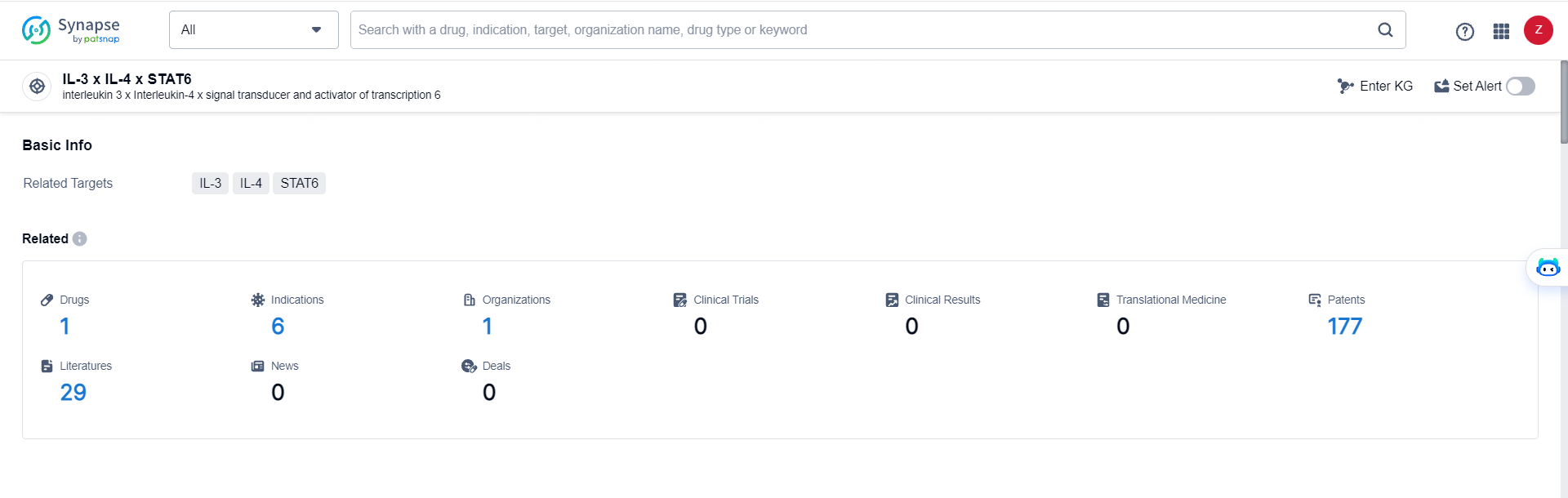
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 28, 2024, there are 1 investigational drugs for the IL-3, IL-4, and STAT6 target, including 6 indications, 1 R&D institutions involved, and as many as 177 patents.
KT-621 targets IL-3, IL-4, and STAT6, making it a potential candidate for the treatment of a wide range of immune system diseases, infectious diseases, congenital disorders, digestive system disorders, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, and skin and musculoskeletal diseases. The active indications for KT-621 include prurigo nodularis, asthma, chronic rhinosinusitis with nasal polyps, dermatitis, eosinophilic esophagitis, and pulmonary disease, chronic obstructive.