Kyowa Kirin Announces Preliminary Phase 3 Results of Rocatinlimab in Moderate to Severe Eczema
Kyowa Kirin Co., Ltd. (Kyowa Kirin) (TSE:4151) (President and CEO: Masashi Miyamoto) disclosed the top-line outcomes from the Phase 3 ROCKET HORIZON study concerning rocatinlimab, a novel therapy aimed at the OX40 receptor. The HORIZON trial successfully reached its co-primary goals: obtaining a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-ADTM) score of 0 (clear) or 1 (almost clear) coupled with a ≥ 2-point reduction from baseline [19.3% rocatinlimab vs. 6.6% placebo (a difference of 12.8%, p<0.001)], along with achieving ≥ 75% reduction in baseline Eczema Area and Severity Index score (EASI-75) [32.8% rocatinlimab vs. 13.7% placebo (a difference of 19.1%, p<0.001)], both at the 24-week mark. Moreover, the trial fulfilled the revised Investigator Global Assessment (rIGA 0/1)*[1], a more precise efficacy metric compared to vIGA 0/1, which uses a stricter definition of 1 (almost clear), at week 24 [16.4% rocatinlimab vs. 4.9% placebo (a difference of 11.5%, p<0.001)].
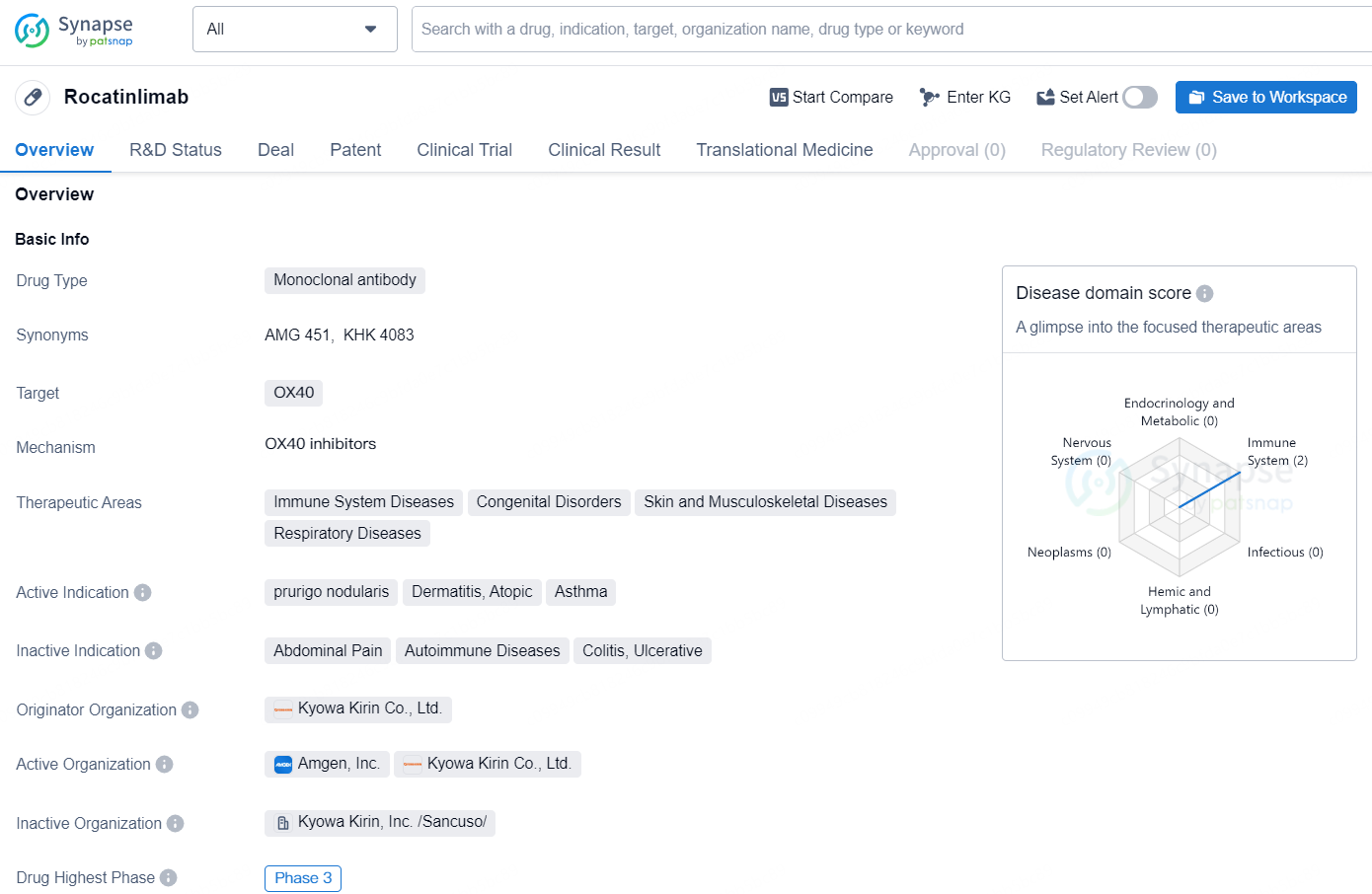
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
HORIZON is a Phase 3, 24-week, randomized, placebo-controlled, double-blind trial designed to evaluate the efficacy, safety, and tolerability of rocatinlimab monotherapy in adult patients with moderate to severe atopic dermatitis (eczema). It is one of the eight trials under the global ROCKET Phase 3 clinical program.
The study has shown statistically significant improvements over placebo across all primary and secondary endpoints. These include measures of skin clearance (vIGA 0/1 and EASI-75 at week 16 and EASI-90 at week 24), the Pruritus Numeric Rating Scale, Atopic Dermatitis Skin Pain Scale, Dermatology Quality of Life Index, and severity scores for hand and facial atopic dermatitis.
"We are delighted with the statistically significant efficacy outcomes compared to placebo, which are consistent across co-primary and all key secondary endpoints. We are excited to further explore rocatinlimab's potential as a T-cell rebalancing therapy for adults with moderate to severe atopic dermatitis, offering a new treatment option. We look forward to obtaining more data from the ROCKET program to fully appreciate the benefits rocatinlimab can provide to patients," said Takeyoshi Yamashita, Ph.D., Senior Managing Executive Officer and Chief Medical Officer at Kyowa Kirin.
Comprehensive results from HORIZON will be presented at a future medical conference. Both Amgen and Kyowa Kirin intend to assess the HORIZON findings and the upcoming results from the other seven ROCKET studies in collaborative discussions with global regulatory authorities.
Kyowa Kirin Group companies are committed to enhancing global health and well-being by generating new value through advancements in life sciences and technology.
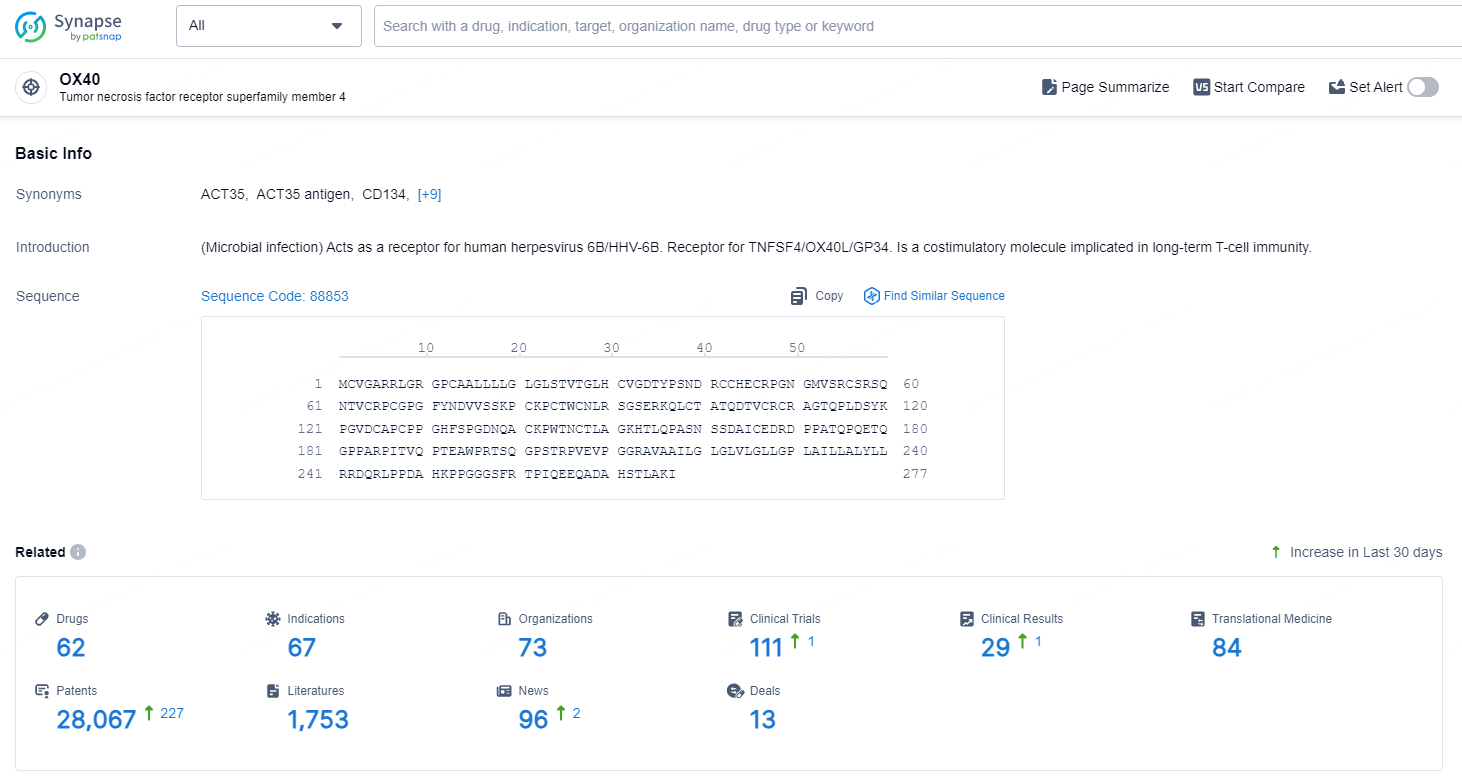
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 62 investigational drugs for the OX40 targets, including 67 indications, 73 R&D institutions involved, with related clinical trials reaching 111, and as many as 28067 patents.
Rocatinlimab is a monoclonal antibody drug that targets OX40. It falls under the therapeutic areas of immune system diseases, congenital disorders, skin and musculoskeletal diseases, and respiratory diseases. The active indications for this drug include prurigo nodularis, dermatitis, atopic, and asthma. The drug is developed by Kyowa Kirin Co., Ltd., a pharmaceutical company known for its focus on biopharmaceuticals and specialty pharmaceuticals.