Merck Updates Trial on Favezelimab-Pembrolizumab Combo for PD-L1+ Metastatic Colorectal Cancer
Merck (NYSE: MRK), referred to as MSD outside the U.S. and Canada, announced that the Phase 3 KEYFORM-007 trial investigating the fixed-dose combination of favezelimab, an anti-LAG-3 antibody developed by Merck, and pembrolizumab (KEYTRUDA®), an anti-PD-1 therapy also by Merck, did not achieve its primary endpoint of overall survival (OS) in patients with previously treated PD-1 positive microsatellite stable (MSS) metastatic colorectal cancer (mCRC). The final pre-specified analysis revealed that the combination of favezelimab and pembrolizumab did not show a survival advantage over the standard of care treatments (regorafenib or TAS-102 [trifluridine and tipiracil hydrochloride]). The safety profile for the combination therapy was consistent with previous studies involving favezelimab and pembrolizumab, and no new safety concerns were identified.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
A comprehensive analysis of the data is currently in progress, and Merck plans to collaborate with researchers to disseminate the findings to the scientific community.
"Treating metastatic colorectal cancer remains a formidable challenge, particularly for the majority of patients with microsatellite stable disease who have shown limited response to immunotherapies," noted Dr. M. Catherine Pietanza, vice president of global clinical development at Merck Research Laboratories. "We extend our gratitude to the patients and investigators for their involvement in this study, and we are committed to advancing our clinical development program to assess KEYTRUDA-based combinations and new candidates for colorectal cancer patients who require additional treatment options."
In the United States, KEYTRUDA is approved for treating patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer as identified by an FDA-approved test. KEYTRUDA is not approved for treating MSS mCRC.
The fixed-dose combination of favezelimab and pembrolizumab is also under investigation for certain hematologic malignancies and various solid tumors. Current studies include KEYFORM-008, a Phase 3 trial assessing the fixed-dose combination in patients with relapsed or refractory classical Hodgkin lymphoma whose disease has worsened after previous anti-PD-1 therapy.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
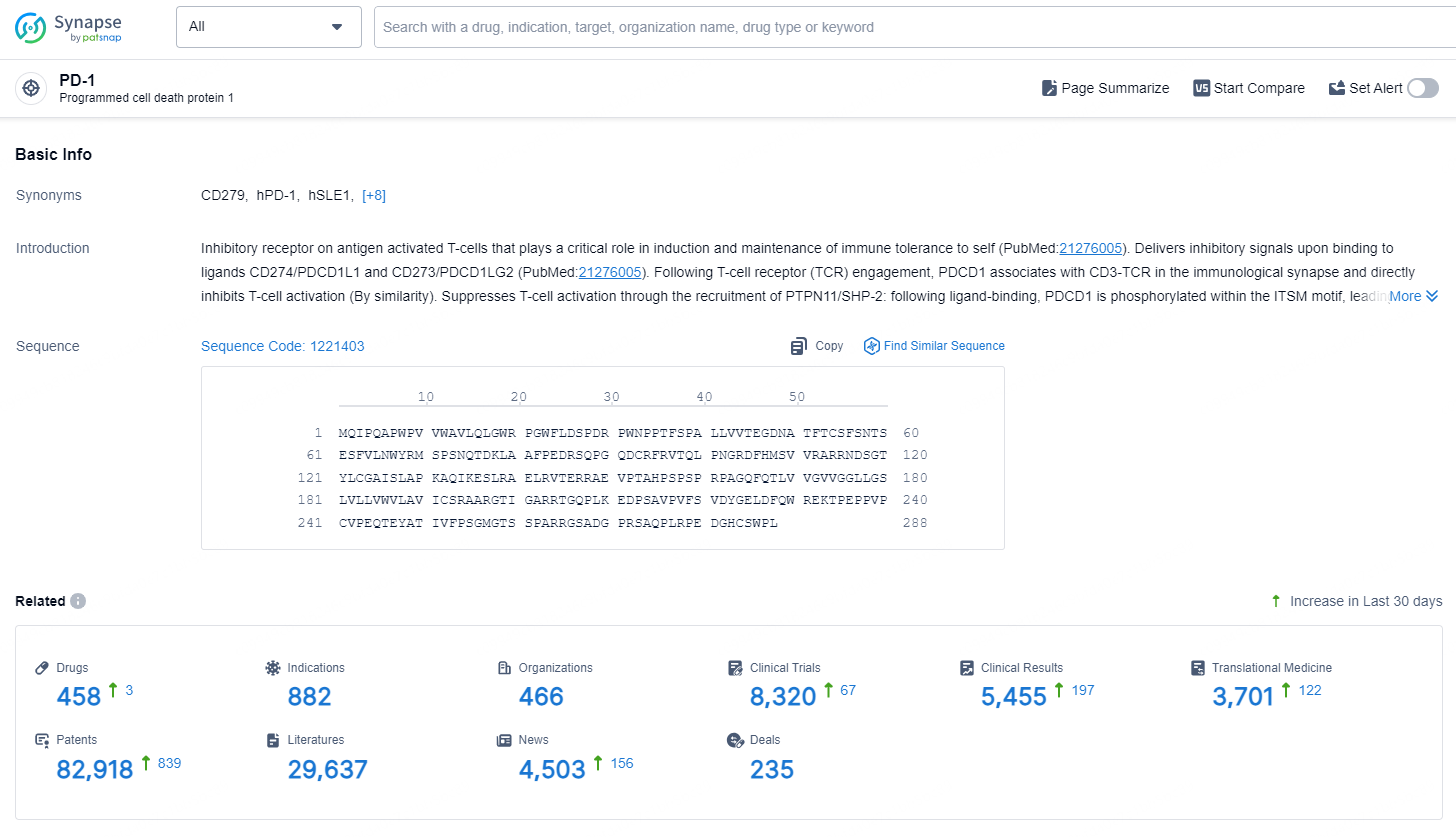
According to the data provided by the Synapse Database, As of September 24, 2024, there are 458 investigational drugs for the PD-1 targets, including 882 indications, 466 R&D institutions involved, with related clinical trials reaching 8320, and as many as 82918 patents.
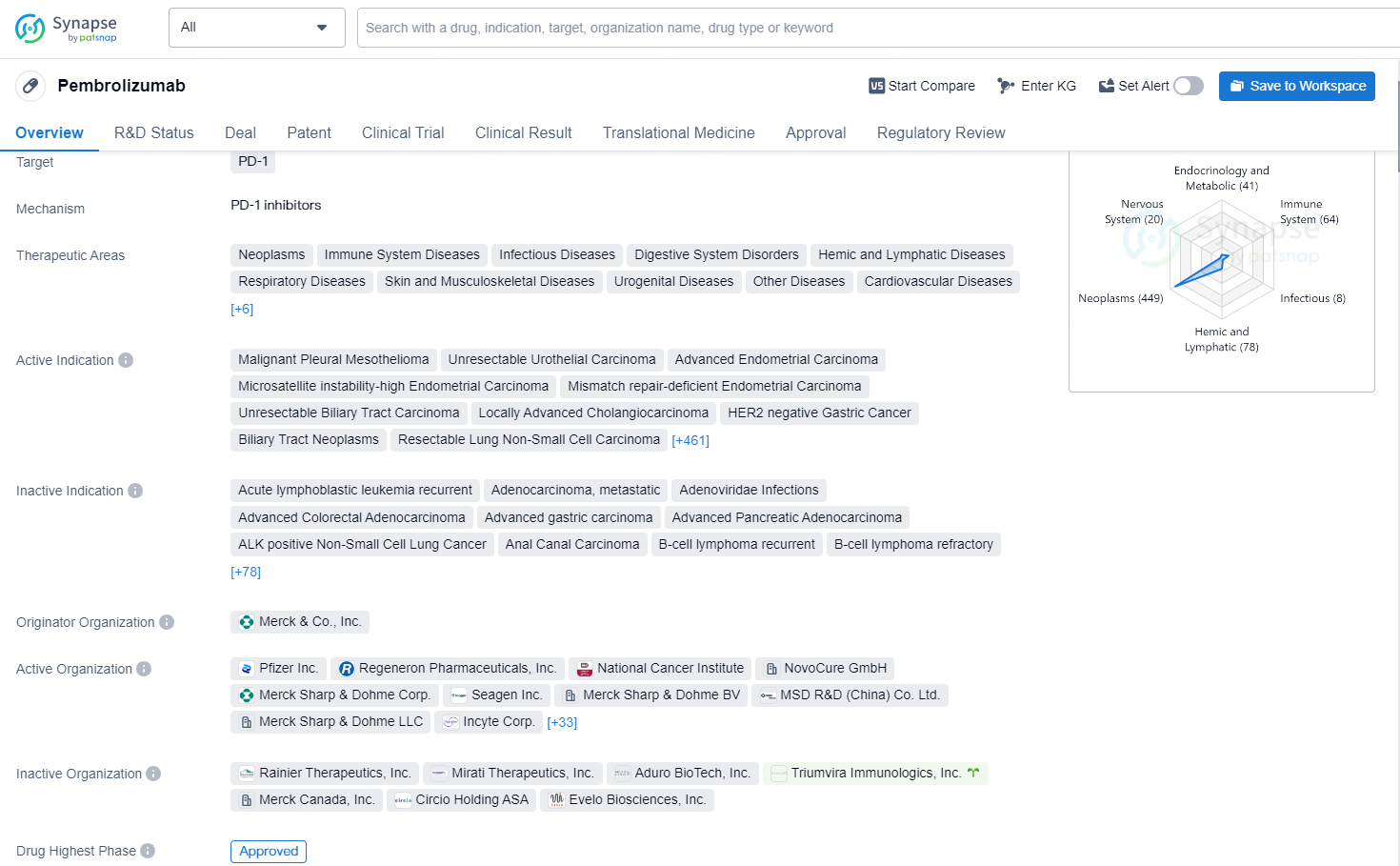
Pembrolizumab is a monoclonal antibody drug that targets the PD-1 receptor and is approved for use in a wide range of therapeutic areas, including neoplasms, immune system diseases, infectious diseases, digestive system disorders, and many others. The drug has been approved for various indications, including malignant pleural mesothelioma, unresectable urothelial carcinoma, advanced endometrial carcinoma, gastric cancer, non-small cell lung cancer, melanoma, and many more.