Lantern Pharma reports initial patient treated in the Phase 1 trial of LP-184 for Advanced Solid Malignancies
Lantern Pharma Inc., an AI firm that creates specialized and revolutionizing cancer treatments through its exclusive AI and machine learning framework, RADR, revealed that it has administered the initial dosage in the Phase 1 clinical trial. The trial will assess Lantern's experimental drug, LP-184, on patients suffering from advanced solid tumors. This move is part of the firm's multiple clinical-stage drug initiatives.
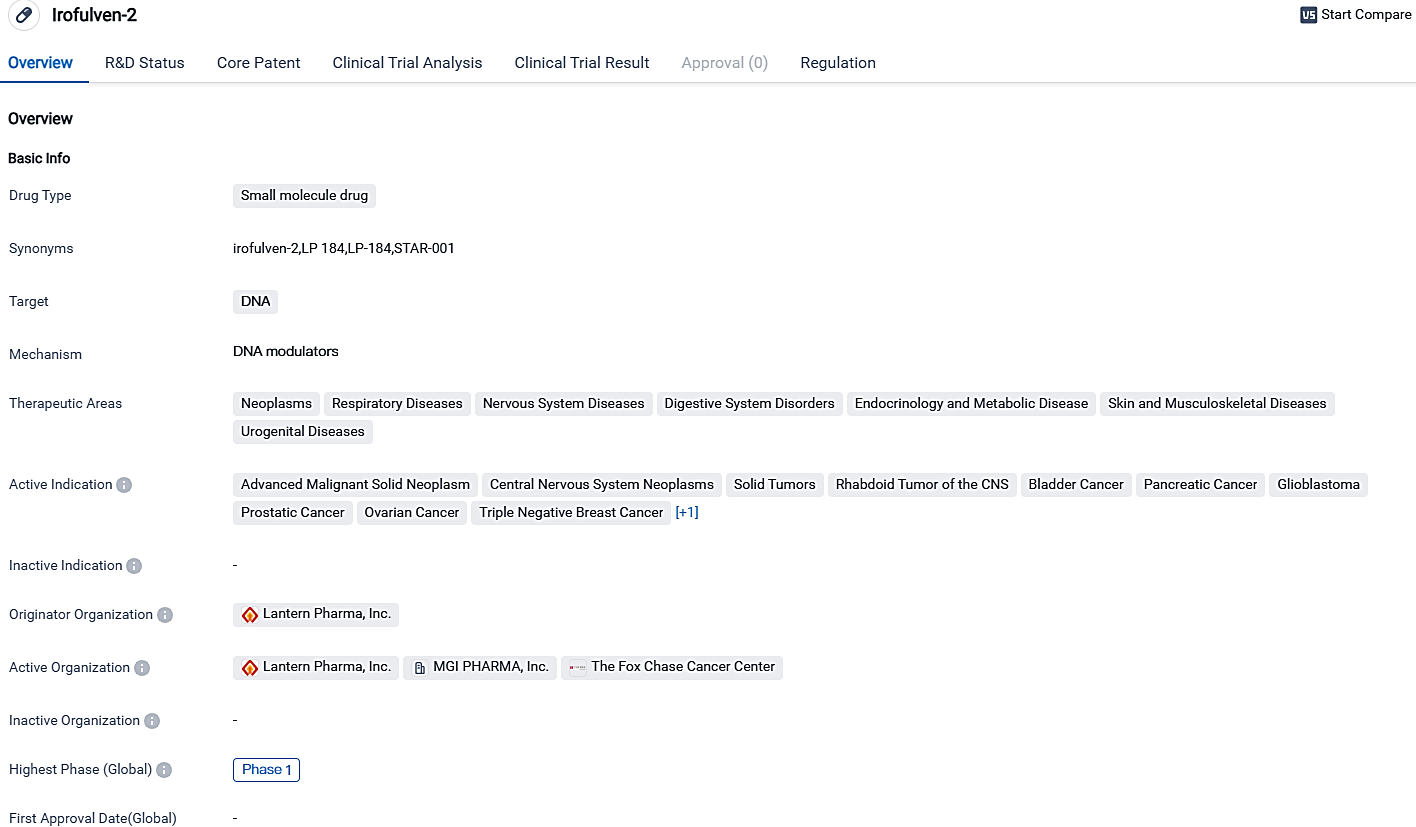
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Our team has achieved a pivotal step in our Phase 1 study of LP-184, symbolizing our unwavering dedication to furthering our strategy aimed at bringing new treatments to patients," said Panna Sharma, President and CEO of Lantern. Panna Sharma added, "This landmark event represents more than just the progression of a unique drug candidate. It reaffirms our distinct strategy of employing AI and machine learning as accelerators in the drug development process. Our own AI and ML platform, RADR®, furnished crucial insights that aided us in creating LP-184 and in producing machine learning biomarker indicators to facilitate patient selection in forthcoming clinical studies."
The sole arm multicentric Phase 1 investigation is evaluating the safety and adaptability of incremented doses of LP-184, with the objective to identify the highest tolerated dose and advise on the Phase 2 dose for patients with advanced solid tumors and recurrent high-grade gliomas, such as glioblastoma.
Upon completion of the Phase 1 study, Lantern intends to progress LP-184 into further clinical testing for various solid tumor conditions. Worldwide, the combined annual market capacity for LP-184/STAR-001 programs is projected to be around $11-13 billion, comprising $6-7 billion for solid tumors and $5-6 billion for CNS malignancies.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
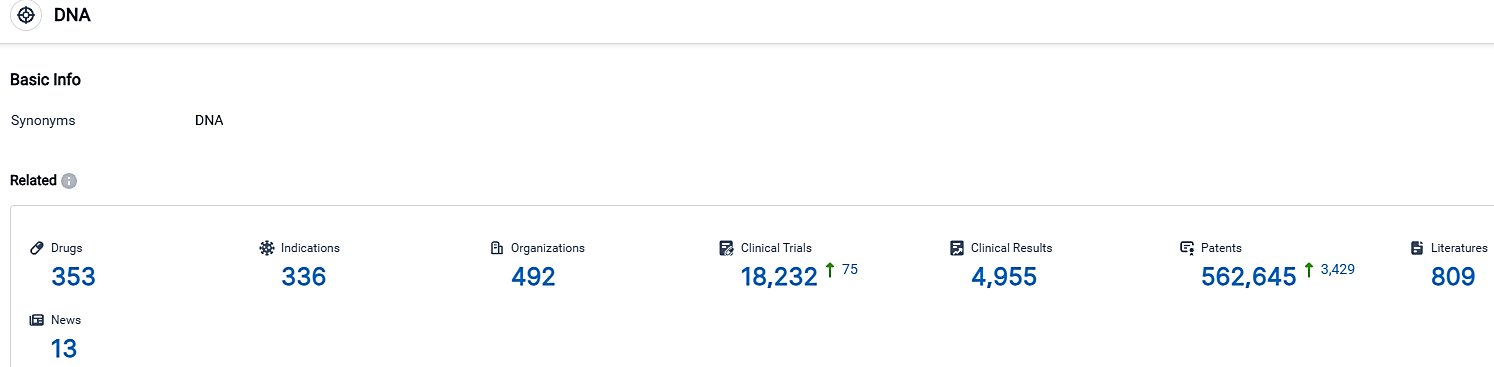
According to the data provided by the Synapse Database, As of September 30, 2023, there are 353 investigational drugs for the DNA target, including 336 indications,492 R&D institutions involved, with related clinical trials reaching 18232,and as many as 562645 patents.
LP-184 is a distinctive minor compound employing a potent operation mode labeled as synthetic lethality, which takes advantage of prevalent weak spots in solid tumor and CNS cancers suffering DNA repair inadequacies. The unique pediatric disease and orphan drug classifications emphasize its ability to meet outstanding healthcare necessities in precise patient cohorts.