Lassen Therapeutics shares new data on LASN01's potential in treating Idiopathic Pulmonary Fibrosis and Thyroid Eye Disease
Lassen Therapeutics, a biotechnology firm actively working in the clinical phase to first-in-class antibody treatments targeting the interleukin-11 receptor (IL-11R, LASN01), is leveraging its potential for dealing with fibro-inflammatory diseases like thyroid eye disease and idiopathic pulmonary fibrosis, as well as using interleukin-18 binding protein as an anticipated cure for cancer. They disclosed new clinical and preclinical findings related to LASN01 obtained at the 2023 European Respiratory Society International Congress along with the 45th Annual Forum of the European Thyroid Association.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"I'm highly enthusiastic about the forthcoming clinical uses empowered by the inhibition of IL-11R in numerous fibro-inflammatory diseases," stated Maria Fardis, PhD, MBA, and CEO at Lassen. "In this regard, we have advanced remarkably in producing data with LASN01 across clinical and non-clinical environments.
We have rounded off our Phase 1 single escalation and multiple escalation dose groups and revealed data from our study involving healthy volunteers at ERS. We also showcased our preliminary data in a TED fibro-inflammatory disease model utilizing orbital fibroblasts at ETA. These OF findings robustly endorse the advancement of LASN01 in TED."
A presentation titled "A Ground-breaking Interleukin-11 Receptor Antibody, LASN01, Demonstrates Good Tolerance and Reflects Target Engagement in Phase 1" detailed Phase 1 information highlighting that LASN01 was well absorbed in healthy volunteers, showcased dose-linear PK, and ended in dose-reliant suppression of STAT3 phosphorylation. The trial is presently accepting idiopathic pulmonary fibrosis and progressive fibrosing interstitial lung disease patients.
The presentation also highlighted the considerable decrease of type VI collagen production (PRO-C6) in human lung tissue treated with LASN01. Administration of LASN01 resulted in a larger reduction of extracellular matrix remodeling as compared to the accepted therapy, nintedanib. LASN01 further displayed a significant antifibrotic effect in a humanized mouse model of IPF lung fibrosis, reducing collagen deposition by over 80%.
OFs were derived from healthy control and TED patients post-orbital decompression surgery and were evaluated for IL-11 expression. IL-11 was found higher in stationary OF received from TED patients in contrast to control cells. The impacts of IL-11R blockade with LASN01 on the discharge of HA, cellular growth, and collagen expression, all catalysts of TED advancement, were explored in reaction to stimulation with IL-11 and other triggers.
IL-11 encouraged reproduction and secretion of HA in OF, both aspects that LASN01 could suppress. Critically, LASN01 also inhibited the combined stimulation of OFs by IGF-1 and IL-11. Moreover, LASN01 performed effectively in combination with teprotumumab, an accepted agent for treating TED, in suppressing HA release.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
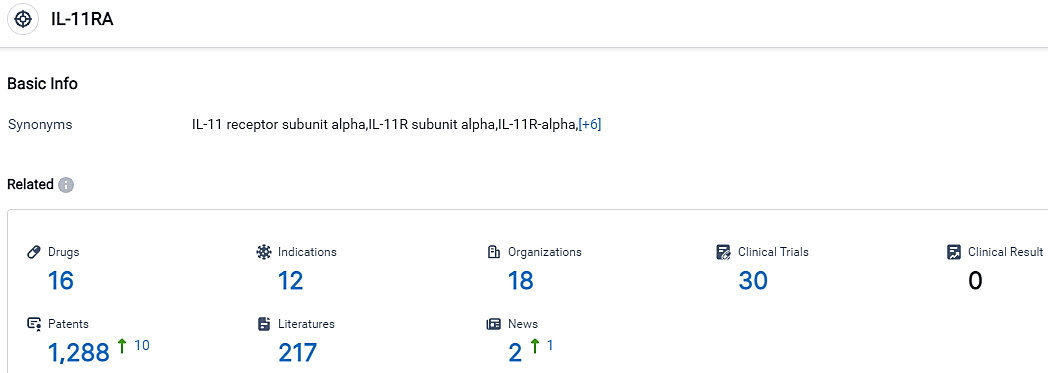
According to the data provided by the Synapse Database, As of September 15, 2023, there are 16 investigational drugs for the IL-11RA target, including 12 applicable indications,18 R&D institutions involved, with related clinical trials reaching 30,and as many as 1288 patents.
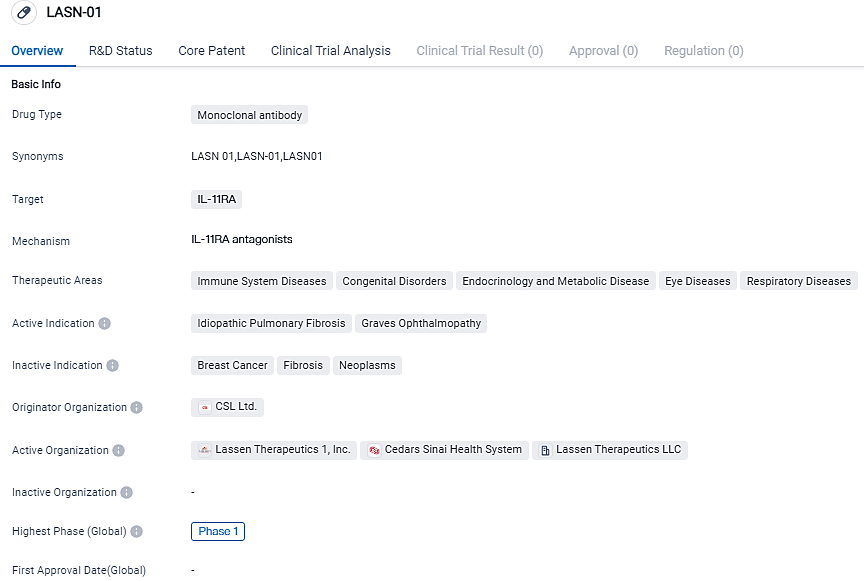
LASN-01 is a monoclonal antibody drug developed by CSL Ltd. that targets IL-11RA. It is being investigated for its potential therapeutic benefits in various diseases, including idiopathic pulmonary fibrosis and Graves ophthalmopathy. Currently in Phase 1 of development, LASN-01 holds promise as a potential treatment option in the field of biomedicine.