Nemesis of Hypercholesterolemia - CETP inhibitors
CETP, or Cholesteryl Ester Transfer Protein, plays a crucial role in lipid metabolism within the human body. It facilitates the transfer of cholesteryl esters (CE) and triglycerides (TG) between high-density lipoproteins (HDL) and low-density lipoproteins (LDL). By promoting the exchange of lipids, CETP contributes to the redistribution of cholesterol and triglycerides among lipoprotein particles. This process is essential for maintaining a healthy lipid profile and preventing the accumulation of cholesterol in the arteries. Understanding the function of CETP has led to the development of pharmaceutical interventions aimed at modulating its activity to improve cardiovascular health.
CETP Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 39 CETP drugs worldwide, from 46 organizations, covering 25 indications, and conducting 173 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.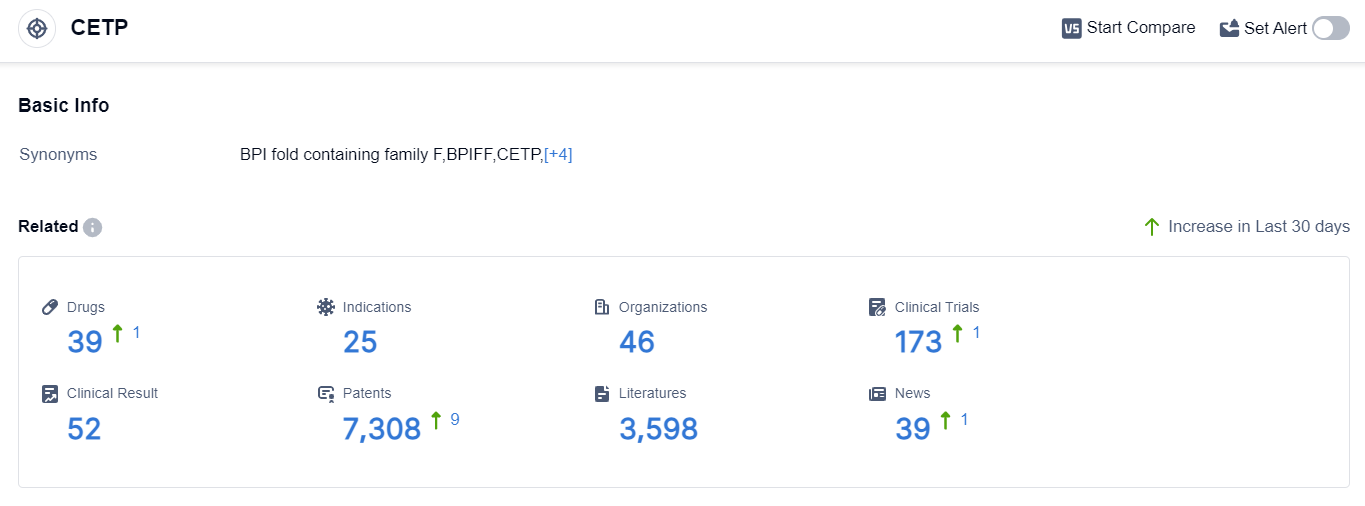
The analysis of target CETP from various perspectives provides valuable insights into the current competitive landscape and future development. Kowa Co., Ltd., NewAmsterdam Pharma Co. NV, and DalCor Pharmaceuticals Canada, Inc. are among the companies with drugs in advanced phases.
Hypercholesterolemia and Hyperlipoproteinemia Type II are the approved indications for target CETP drugs. Small molecule drugs dominate the development landscape, with intense competition indicated by the presence of biosimilars.
Japan, the United States, and the European Union are leading in terms of development, with China also making progress. Overall, the target CETP shows promising potential for addressing various indications related to cardiovascular diseases and lipid metabolism disorders.
CETP Inhibitor Entering Phase III Clinical Trials:Obicetrapib
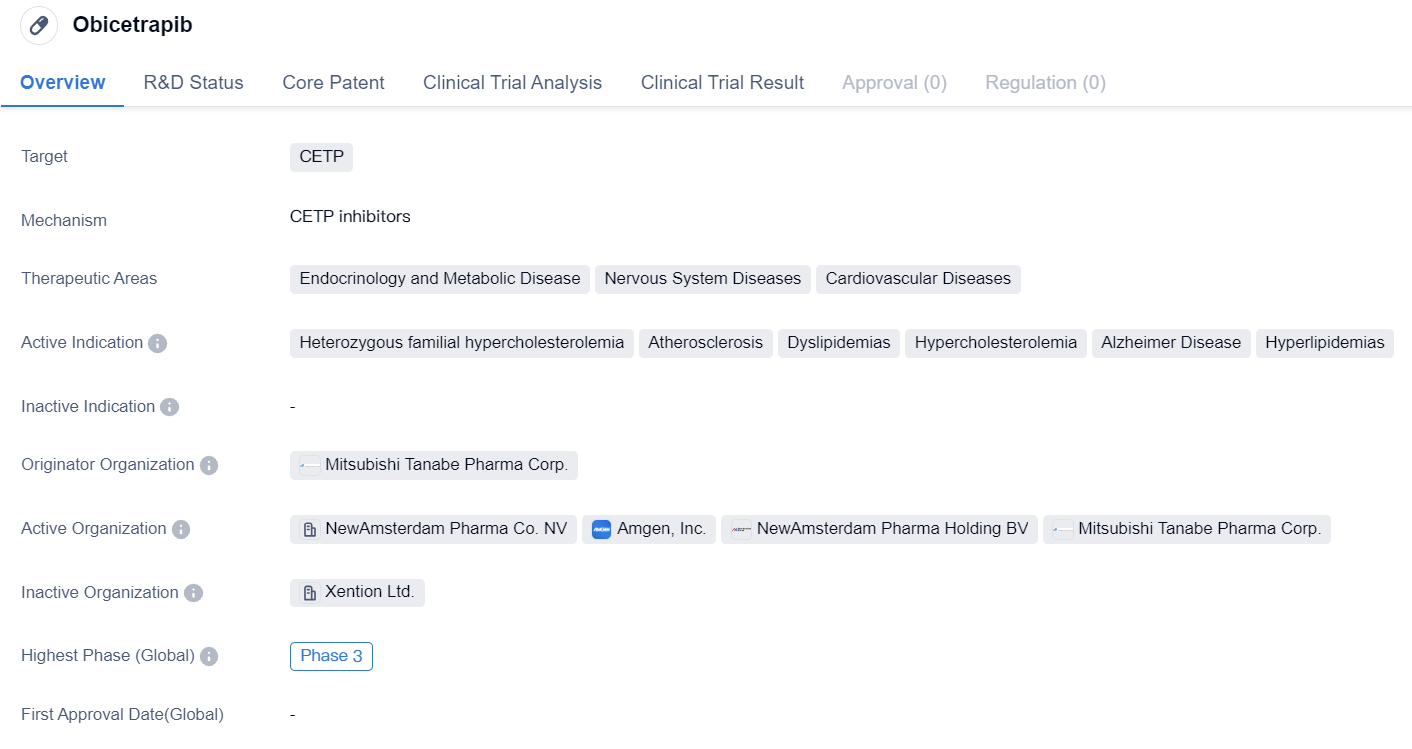
Obicetrapib is a small molecule drug that falls under the category of biomedicine. It specifically targets CETP. The drug is primarily focused on treating various therapeutic areas including Endocrinology and Metabolic Disease, Nervous System Diseases, and Cardiovascular Diseases.
Obicetrapib has been identified to be effective in treating several active indications. These include Heterozygous familial hypercholesterolemia, Atherosclerosis, Dyslipidemias, Hypercholesterolemia, Alzheimer Disease, and Hyperlipidemias. These indications highlight the drug's potential in managing cholesterol-related disorders and neurodegenerative conditions such as Alzheimer's disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization responsible for the development of Obicetrapib is Mitsubishi Tanabe Pharma Corp. This pharmaceutical company has been actively involved in the research and development of this drug.
In terms of clinical development, Obicetrapib has reached Phase 3 of clinical development globally. This indicates that the drug has undergone extensive preclinical testing and has progressed to advanced stages of clinical trials involving a larger patient population. Phase 3 trials are crucial in evaluating the drug's safety, efficacy, and dosage requirements before seeking regulatory approval. Similarly, in China, Obicetrapib has also reached Phase 3 of clinical development. This suggests that the drug's development has progressed to an advanced stage in the Chinese market as well.
In summary, Obicetrapib is a small molecule drug developed by Mitsubishi Tanabe Pharma Corp. It targets CETP and shows potential in treating various therapeutic areas such as Endocrinology and Metabolic Disease, Nervous System Diseases, and Cardiovascular Diseases. The drug has active indications including Heterozygous familial hypercholesterolemia, Atherosclerosis, Dyslipidemias, Hypercholesterolemia, Alzheimer Disease, and Hyperlipidemias. With its highest phase being Phase 3 globally and in China, Obicetrapib has undergone extensive clinical development and is currently being evaluated for its safety and efficacy in larger patient populations.
Evacetrapib
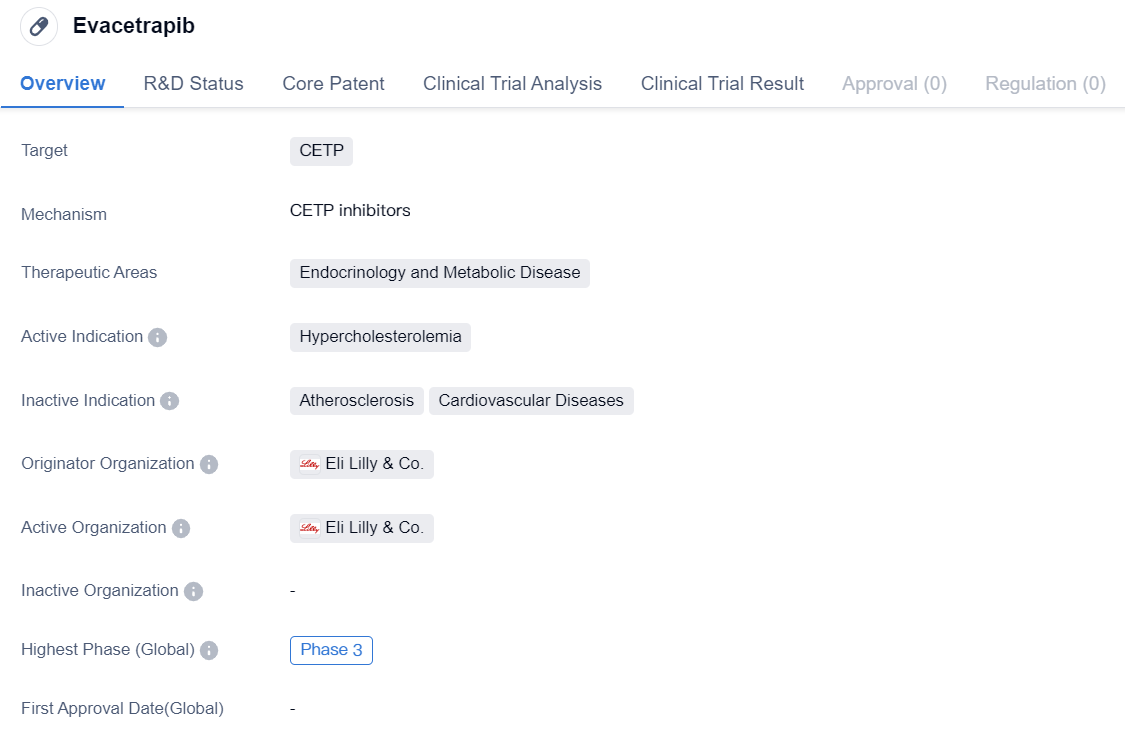
Evacetrapib is a small molecule drug that is being developed to target CETP (Cholesteryl Ester Transfer Protein). It falls under the therapeutic areas of endocrinology and metabolic disease, specifically focusing on the treatment of hypercholesterolemia, which is characterized by high levels of cholesterol in the blood. The drug is being developed by Eli Lilly & Co., a renowned pharmaceutical company.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As of the latest available information, Evacetrapib has reached Phase 3 of clinical development, on a global scale. Phase 3 trials are conducted to evaluate the safety and efficacy of a drug in a larger population, typically involving thousands of patients. This indicates that Evacetrapib has successfully progressed through earlier stages of development, including preclinical studies and Phase 1 and 2 trials.
However, it is important to note that the highest phase of development for Evacetrapib in China is listed as "discontinued." This suggests that the drug's development in China has been halted or terminated at some point, possibly due to various reasons such as lack of efficacy, safety concerns, or strategic decisions by the originator organization.
In summary, Evacetrapib is a small molecule drug developed by Eli Lilly & Co. that targets CETP and is intended for the treatment of hypercholesterolemia. It has reached Phase 3 of clinical development globally, indicating promising progress in its development. However, in China, the drug's development has been discontinued, suggesting potential challenges or setbacks specific to the Chinese market.