Latest Research Progress on TLR Adjuvants
The TLR agonists used in vaccine formulations come in various forms, ranging from lipopeptides to single-stranded DNA and RNA. Below, we will introduce the main categories of TLR adjuvants.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
TLR2
TLR2 is expressed on the surface of various cells, including monocytes, macrophages, endothelial cells, epithelial cells, natural killer cells, dendritic cells, myeloid-derived suppressor cells, platelets, and mast cells. The PAMP library recognized by TLR2 is the most extensive, as it can form heterodimers with TLR1 and TLR6 (and TLR10 in humans).
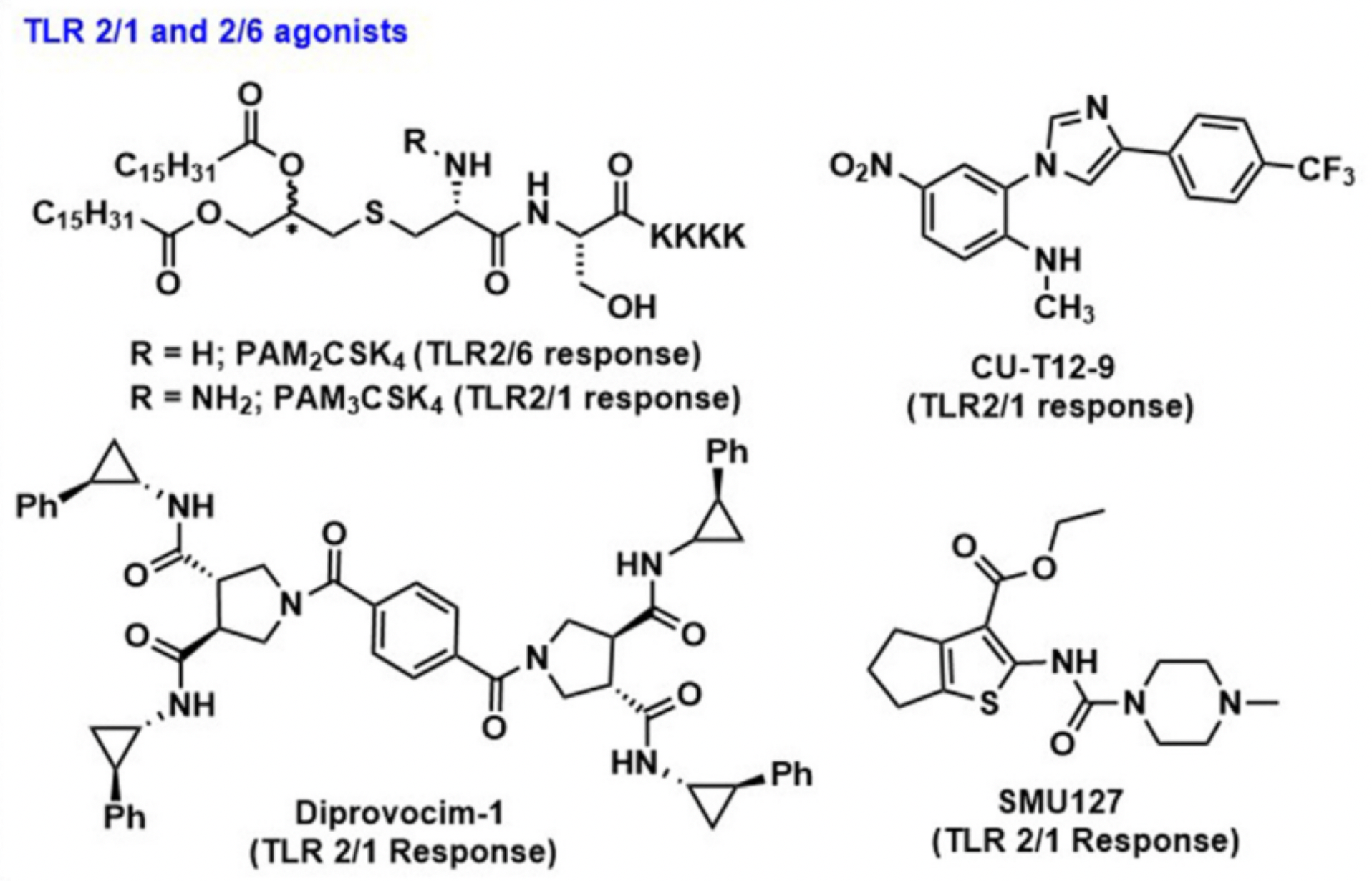
The range of TLR2 adjuvants is extensive, including synthetic lipopeptides (PAM3CSK4 and PAM2CSK4), arabino-mannose lipids, phospholipidic acid, GPI membrane anchors, yeast polysaccharides, and peptidoglycans. Recent trends in TLR2 research include the discovery of small synthetic TLR2 agonists, improvements in the characteristics of traditional TLR2 agonists, and the bio-conjugation of TLR2 with vaccine antigens. For example, XS15 (PAM3CS-GDPKHPKSF) is a new TLR-1/2 agonist based on PAM3CS, where the four lysines (K4) of PAM3CSK4 are replaced with a nonapeptide (GDPKHPKSF) to alter the solubility of the conjugate, promoting uptake and facilitating purification. Constraints of using TLR2 agonists as adjuvants include the size, complexity, and hydrophobicity of most ligands.
TLR3
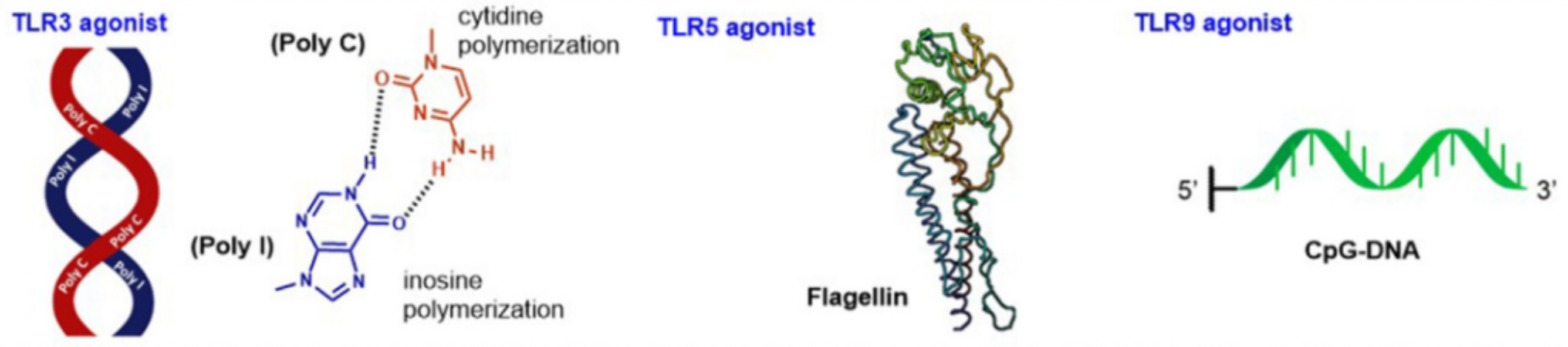
TLR3 is an intracellular recognition system that reacts to viral nucleic acids (dsRNA, ssRNA, and ssDNA) and endogenous double-stranded RNA. Many TLR3 agonists have been developed, such as RGC100 and ARNAX, a synthetic DNA-RNA hybrid compound. However, in the past two years, researchers have returned to using traditional dsRNA analog ligands, such as poly IC, focusing on improving their delivery modes and new disease applications.
TLR4
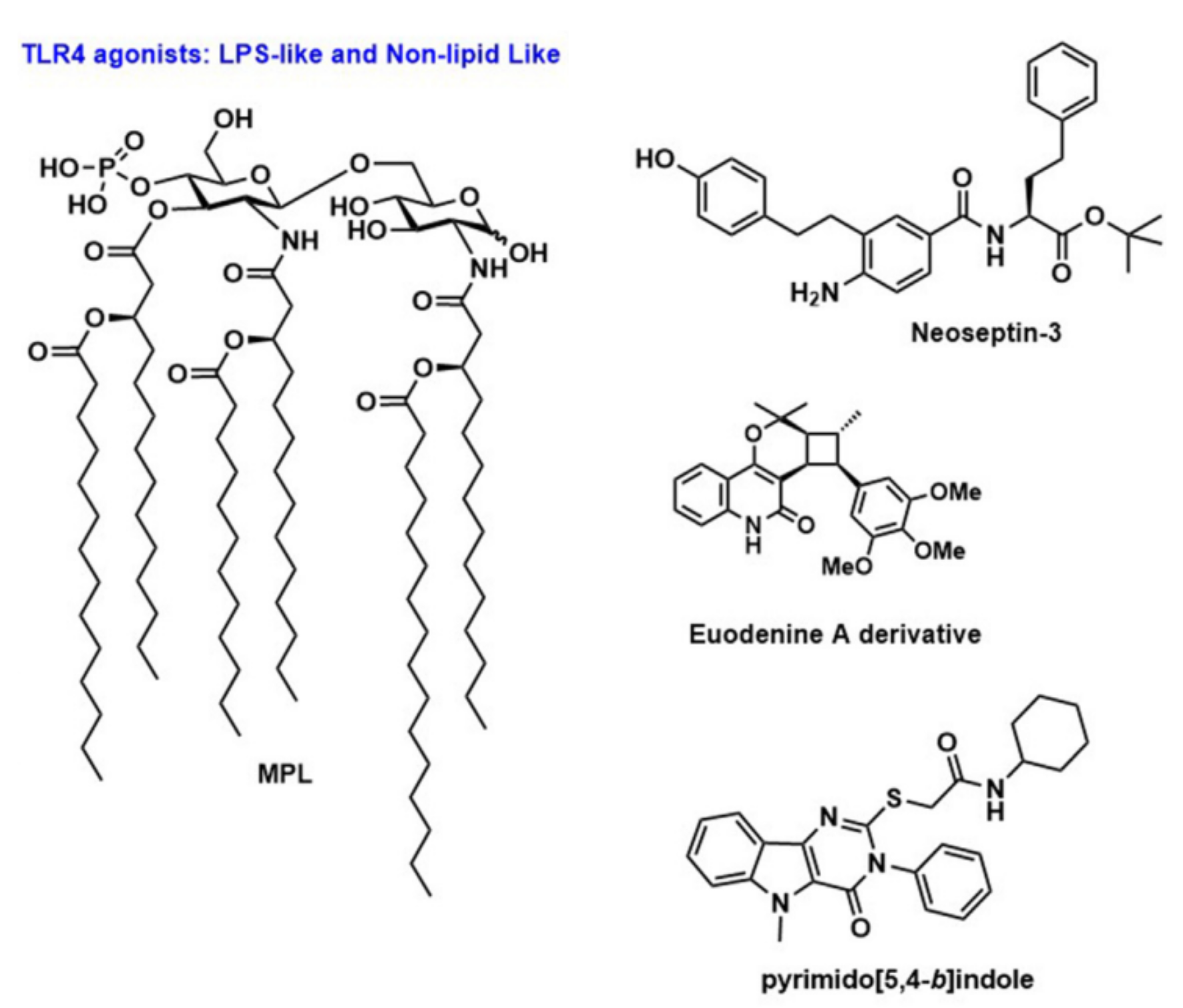
TLR4 is the most studied member of the TLR family, recognizing lipopolysaccharides (LPS). TLR4 is located on the cell membrane, mainly expressed on myeloid cells, but not on plasmacytoid dendritic cells (pDCs) and immature B cells. TLR4 recognizes LPS through its co-receptors myeloid differentiation factor-2 (MD-2) and CD14. Recent works on TLR4 agonists concentrate on the development and evaluation of modified products, such as monophosphoryl lipid A (MPLA) and Glucopyranosyl lipid A (GLA), which are structurally related to LPS, but have no high pyrogenicity while maintaining strong immunoenhancing characteristics, thus increasing their clinical feasibility.
TLR5
TLR5 recognizes flagellin protein and is expressed on epithelial cells and immune cells (such as macrophages and immature DC), generating immune responses through the MyD88-dependent signaling pathway. Recent work on TLR5 agonists has mainly focused on enhancing tolerance. Unfortunately, flagellin can induce unnecessary hyperreactivity, as a protein, it can induce antibodies against itself, interfering with its function as an adjuvant. This issue has been solved by research through removing the B cell epitope region from flagellin, the deimmunized flagellin retains its TLR5 adjuvant activity.
TLR7/8
TLR7 and TLR8 are located on the endosomal membrane of immune cells. TLR7 is primarily expressed in pDC and B cells, while TLR8 is expressed in myeloid dendritic cells and monocytes, with lower expression in pDC. TLR7/8 recognizes single-strand ribonucleic acid (ssRNA), and signals through the MyD88-dependent pathway. Among natural ligands, oligonucleotides (ORNs) enriched with adenine and uridine can activate TLR8 without any effect on TLR7. However, ORNs enriched in guanine activate TLR7 and TLR8-dependent signal transduction. TLR7 exists as a monomer and dimerizes in the presence of ligand, while TLR8 exists as weakly dimerized in its natural state and undergoes conformational change when ligand binds. To date, more than15 novel heterocyclic molecules, such as imidazoquinoline, oxazine, pyrimidine, pyridopyrimidine, pyrrolopyrimidine and benzimidazole, have been identified as TLR7/8 agonists.
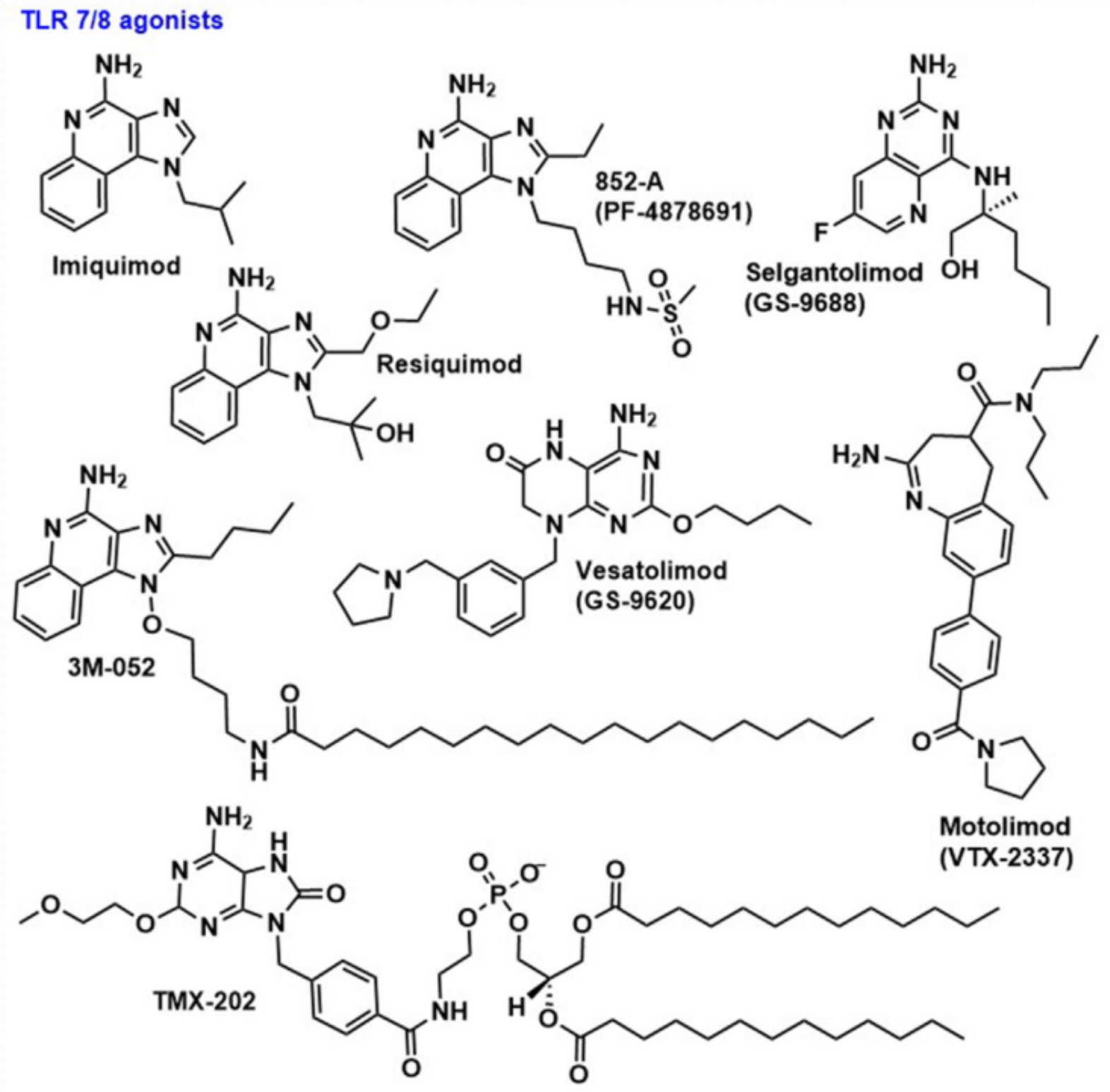
A common drawback of TLR7/8 agonists is their reactogenicity, and in recent years, numerous studies have attempted to overcome these adverse effects. Encapsulating TLR7/8 agonists in nanoparticles based on cationic DOEPC liposome formulations, or covalently linking these small molecules to hyperbranched polymers, can avoid their harmful systemic reactions while preserving their impact on humoral immunity.
TLR9
TLR9 is located within the endosomal membrane in cells, recognizing the single-stranded unmethylated CpG oligonucleotides of bacteria and virus DNA. TLR9 is expressed on immune cells such as dendritic cells, macrophages, natural killer cells, and other APCs. Synthetic CpG sequences can be used as TLR9 agonists to enhance the immune response of vaccines, and each unique sequence variant combination has been proven to have different structural and biological characteristics.
The latest developments in TLR9 agonists in the past two years have mainly focused on the efficient delivery and uptake of CpG to cells. For example, one study introduced a new method of conjugating CpG-ODN to novel cationic liposomes, this compound can induce a strong immune response at low antigen and adjuvant doses.
Combined TLR Adjuvants
Combining different TLR agonists in a single vaccine can generate a synergistic effect, driving potent vaccine immune responses. For example, liposomal adjuvants containing 1V270 (TLR7 agonist) and 2B182C (TLR4 agonist) induced balanced anti-HA and anti-NA IgG1 and IgG2a responses against influenza, without the overreactivity commonly associated with Th1 proinflammatory responses. Similarly, one study assessed the co-encapsulation of ovalbumin (OVA) with ten unique combinations of two to three TLR ligands, including Pam3CSK4 (TLR2 agonist), MPLA (TLR4 agonist), imiquimod (TLR7/8 agonist) and CpG (TLR9 agonist), with triple combinations promoting antigen-specific antibody titers through a balanced overall Th1/Th2 response. Therefore, combinations of TLR adjuvants can offer broad customizability of immune responses.
Combinations of TLR agonists with other PAMPs such as NOD2 and macrophage-inducible Ca²⁺-dependent lectin receptor (Mincle) ligands are also promising. Covalent linkage of CL239 (TLR7 agonist) and muramyl dipeptide (NOD2 agonist), and incorporation of the dual agonist as nanoparticles with the NP-p24 HIV vaccine, synergistically enhanced protection in mice.
Summarize
TLR agonists utilize endogenous innate immune pathways to enhance vaccine adaptive immune responses. Over the past two years, the domain of adjuvants has been primarily governed by research into the COVID-19 vaccine. The driving force of this pandemic has rapidly promoted the development of the adjuvant field, and several novel adjuvants have now been incorporated into licensed vaccines for COVID-19. Beyond COVID-19, the demand for more effective adjuvants continues to grow, particularly in the areas of difficult-to-prevent infectious diseases such as cancer, malaria, tuberculosis, and HIV. Unraveling the mechanisms behind the action of TLR co-adjuvants, coupled with increased safety and efficacy research data, can help drive the development of the next generation of adjuvant platforms.