Lilly to Buy Out Morphic to Enhance Treatment Options for Inflammatory Bowel Disease Patients
Eli Lilly and Company along with Morphic Holding, Inc. have revealed a formal agreement in which Lilly will purchase Morphic, a biopharmaceutical firm focused on creating oral integrin therapies aimed at managing severe chronic illnesses.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
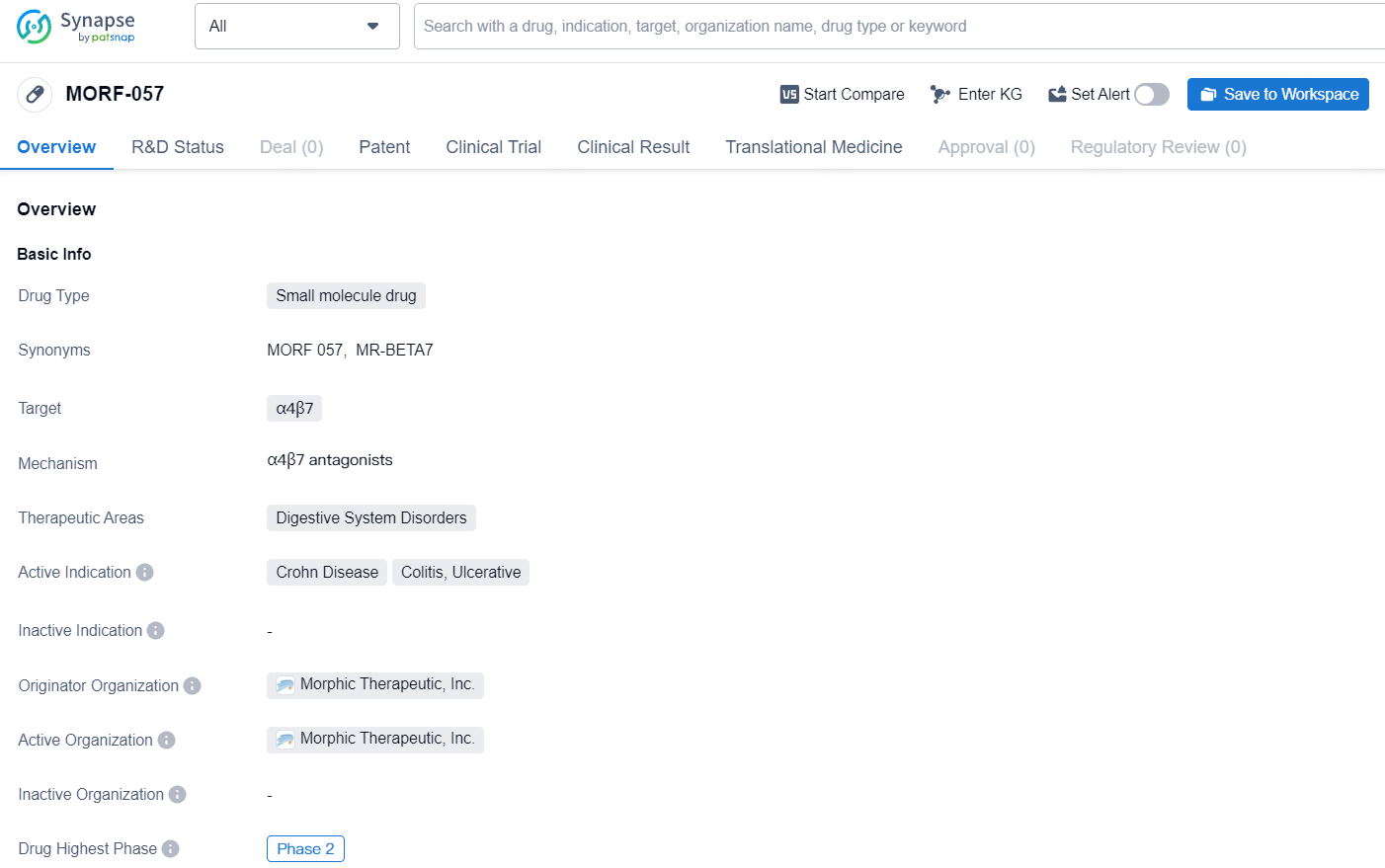
Morphic's primary initiative focuses on an oral, small molecule inhibitor specifically targeting the α4β7 integrin for inflammatory bowel disease treatment. This therapeutic candidate, MORF-057, has the potential to enhance patient outcomes and broaden available treatment options. Currently, MORF-057 is undergoing assessment in two Phase 2 trials for ulcerative colitis and one Phase 2 trial for Crohn’s disease. Beyond MORF-057, Morphic is advancing a preclinical portfolio that includes compounds for autoimmune diseases, pulmonary hypertension, fibrotic conditions, and cancer.
According to Daniel Skovronsky, M.D., Ph.D., chief scientific officer at Lilly and president of Lilly Research Laboratories and Lilly Immunology, "Oral treatments could present new opportunities for early intervention in diseases such as ulcerative colitis. These therapies might also allow for combination treatments to aid patients with more severe conditions. We are excited to integrate Morphic's team into Lilly, as this strategic acquisition demonstrates our dedication to developing innovative gastroenterology therapies. Lilly has made substantial investments to introduce pioneering molecules that benefit patients."
Praveen Tipirneni, M.D., CEO of Morphic Therapeutic, stated, "Morphic has consistently believed in the significant potential of MORF-057 to aid patients with IBD, a potential best realized through an ideal strategic partner. Lilly provides unmatched expertise and dedication to the inflammation and immunology sectors. Our Morphic Integrin Technology platform was established to harness the extensive possibilities of integrin-based therapies. MORF-057 exemplifies these efforts, given its profile as a well-tolerated, effective oral small molecule α4β7 inhibitor, which could pave the way for novel IBD treatments. My heartfelt gratitude goes to the entire Morphic Team for their skill, innovation, and perseverance. We also thank the investigators and patients who have played a crucial role in MORF-057's progress so far. We look forward to the future development of MORF-057 and other integrin therapies under Lilly’s guidance."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
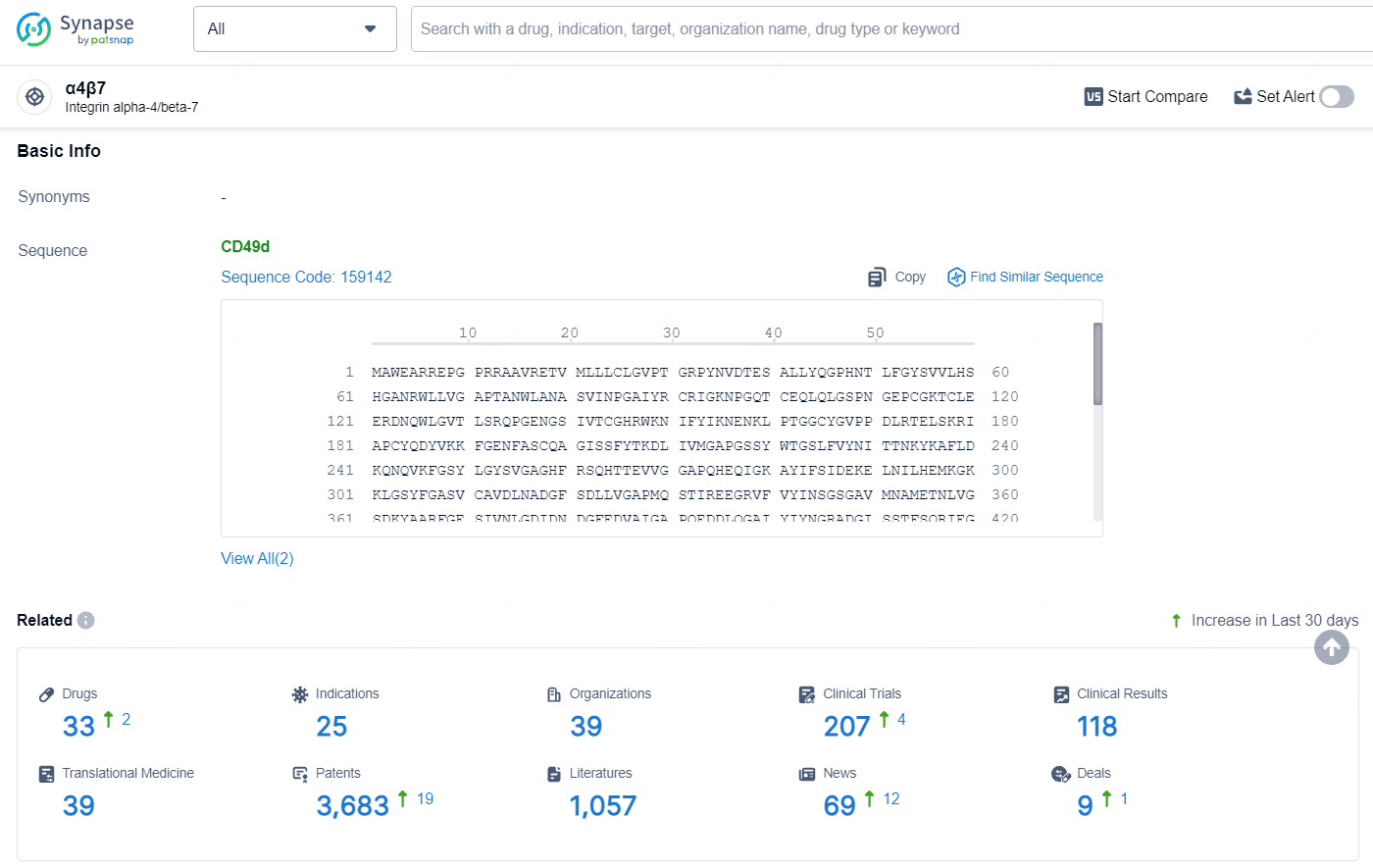
According to the data provided by the Synapse Database, As of July 10, 2024, there are 33 investigational drugs for the α4β7 targets, including 25 indications, 39 R&D institutions involved, with related clinical trials reaching 207, and as many as 3683 patents.
MORF-057 is a small molecule drug developed by Morphic Therapeutic, Inc., targeting α4β7 and focusing on treating Crohn Disease, Colitis, and Ulcerative. With its current status in Phase 2 of clinical trials, MORF-057 holds promise as a potential therapeutic option for patients with digestive system disorders.