Ligand is set to purchase APEIRON Biologics AG in a deal valued at $100 million
Ligand Pharmaceuticals Incorporated revealed that it has finalized a definitive contract to purchase APEIRON Biologics AG. This acquisition includes the royalty rights to QARZIBA (dinutuximab beta), a treatment for high-risk neuroblastoma, for a total cash transaction of $100 million. Additionally, Ligand will provide APEIRON shareholders with further payments contingent on upcoming commercial and regulatory developments, consisting of up to $28 million if QARZIBA royalties surpass specific predetermined limits by either 2030 or 2034.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 APEIRON is a Vienna-based private biopharmaceutical firm. The company played a key role in the development of QARZIBA, which is used to treat high-risk neuroblastoma in patients aged 12 months and older. QARZIBA received approval from the European Medicines Agency in 2017 and is now available in more than 35 countries.
APEIRON is a Vienna-based private biopharmaceutical firm. The company played a key role in the development of QARZIBA, which is used to treat high-risk neuroblastoma in patients aged 12 months and older. QARZIBA received approval from the European Medicines Agency in 2017 and is now available in more than 35 countries.
APEIRON earns an undisclosed royalty on QARZIBA's net sales outside of mainland China through Recordati S.p.A, a leading global pharmaceutical company operating in over 150 countries with annual sales exceeding $2.2 billion. Additionally, they receive royalties on net sales within mainland China from BeiGene, Ltd.
Todd Davis, CEO of Ligand, stated, “Incorporating QARZIBA into our commercial royalty portfolio aligns with our strategy of investing in high-value medicines that offer substantial clinical benefits and generate predictable, long-term revenue streams for our investors. QARZIBA remains the sole immunotherapy for high-risk neuroblastoma available across Europe and other regions. We anticipate this drug will significantly contribute to our royalty revenue, which is now supported by a diversified portfolio of 12 key commercial-stage products.”
Peter Llewellyn-Davies, CEO of APEIRON, said, “This deal represents a significant landmark for our company and shareholders. Over the past 20 years, we have worked diligently to convert academic research into therapeutic products for diseases with high unmet needs. Our team is grateful to have contributed to making QARZIBA accessible to the young patients in need of this treatment. We value Ligand’s recognition of the long-term potential of this vital medication for a rare pediatric cancer.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
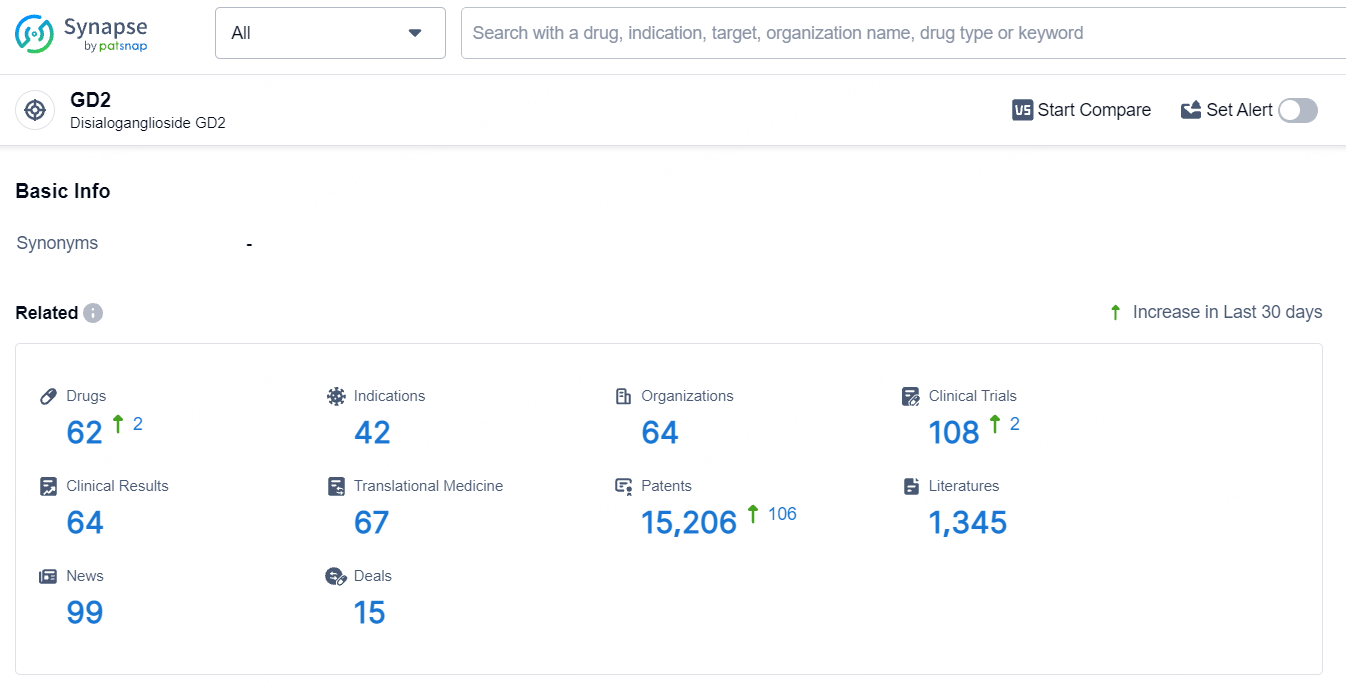
According to the data provided by the Synapse Database, As of July 10, 2024, there are 62 investigational drugs for the GD2 target, including 42 indications, 64 R&D institutions involved, with related clinical trials reaching 108, and as many as 15206 patents.
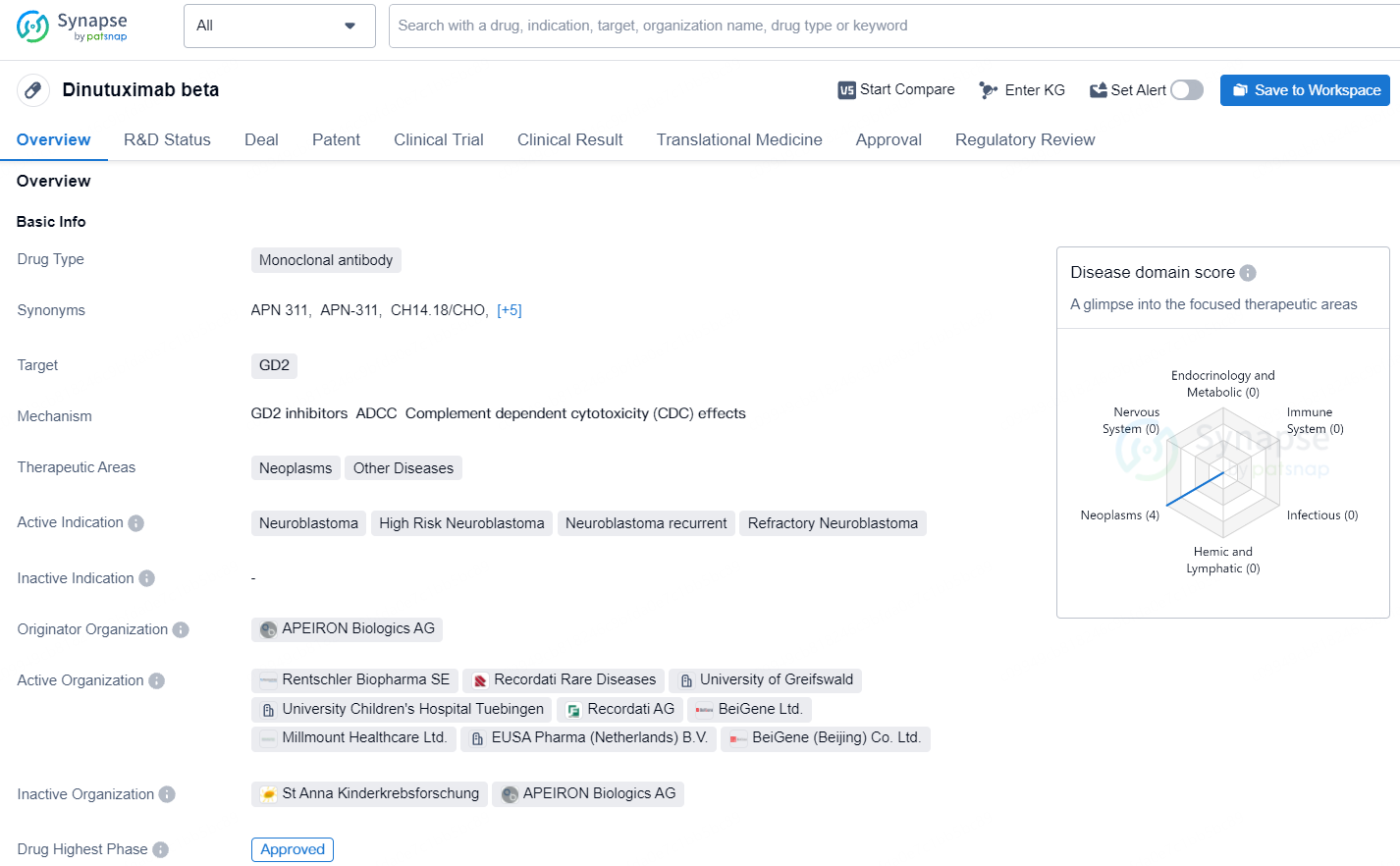
Dinutuximab beta is a monoclonal antibody drug that targets GD2 and is used in the treatment of neoplasms and other diseases. Dinutuximab beta has received approval in multiple countries, including the European Union, and has been granted various regulatory designations to expedite its availability for patients in need. As an approved drug with a specific target and therapeutic areas.